CASE III: P5952-17 (JPC 4118012)
Signalment:
Adult (exact age not specified but assessed as 2-3 year-old by veterinarian), female, crossbred, Capra aegagrus hircus, domestic goat
History:
This goat was culled for undisclosed reasons and sent to the abattoir. Although the body condition was suboptimal, this goat was assessed as fit for slaughter at the ante-mortem examination.
Gross Pathology:
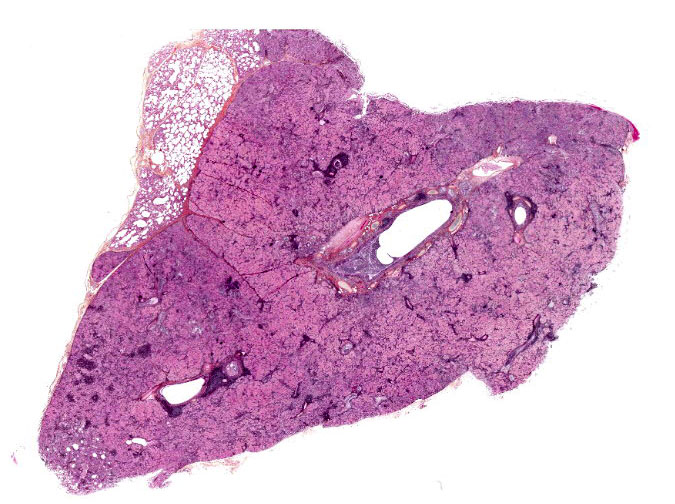
The submitted post-mortem examination report only mentioned abnormal lungs. They were described by the veterinarian as diffusely mottled with numerous firm, gray to pale red consolidated areas. On cut section, there was a thick mucopurulent exudate in the bronchi of the anterior portions of both lungs. Fresh and formalin-fixed samples from all lobes were sent to our laboratory.
Laboratory Results:
Immunohistochemistry was multifocally strongly positive for small ruminant lentivirus (SRLV à MMV and CAEV).
Microscopic Description:
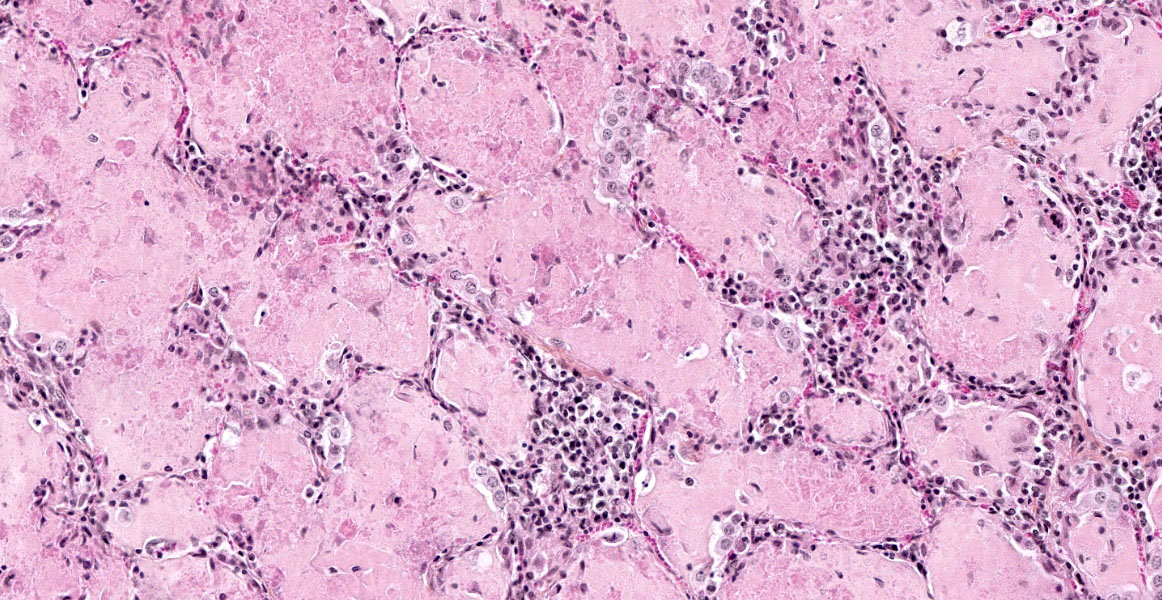
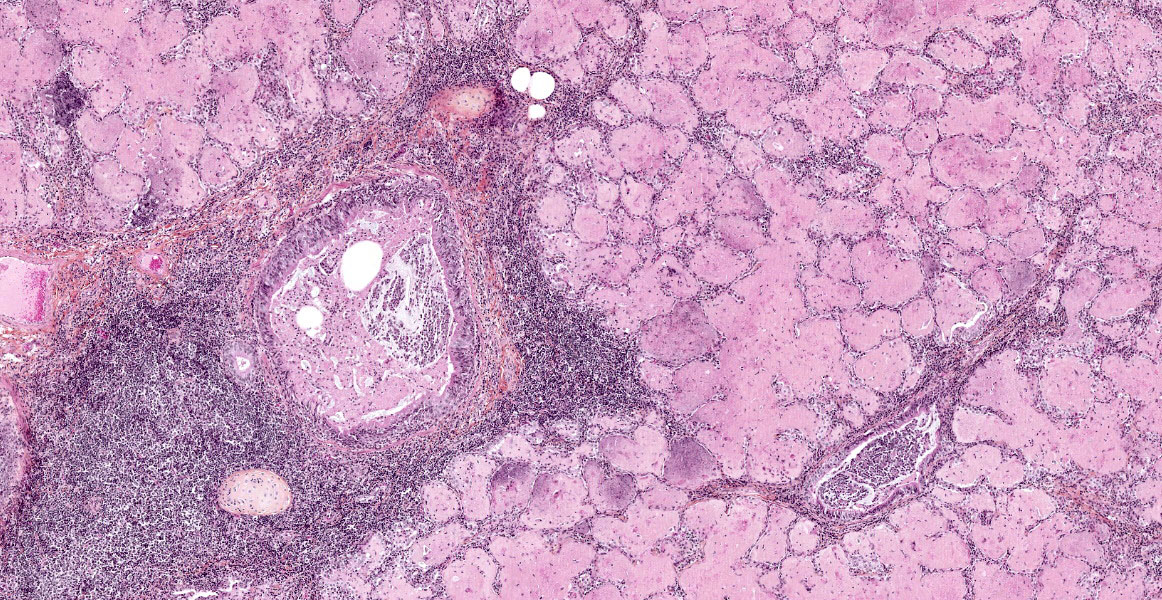
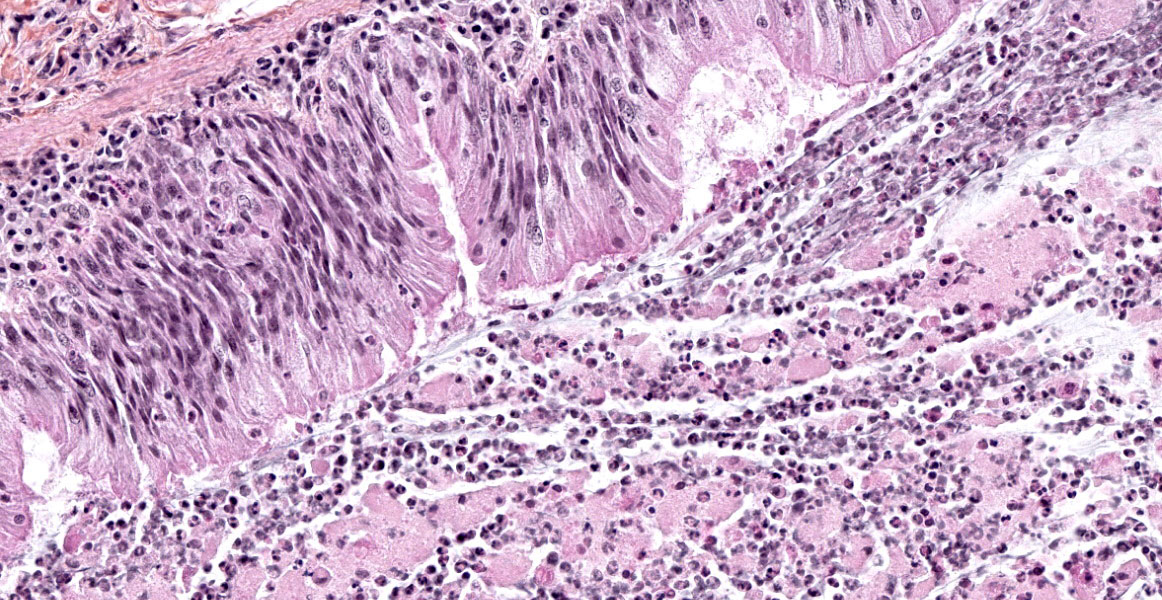
The lesions are similar in all pulmonary lobes (only one section submitted). The lesions are lobular, with normal lobules adjacent to pneumonic ones. In affected lobules, there is a lymphoplasmacytic infiltrate variably thickening the airway and vascular adventitia, and to a lesser degree the alveolar septa (interstitial pneumonia). Multifocally, the alveoli are lined by usually plump type II pneumocytes (proliferative pneumonia). The alveoli are filled by dense amorphous eosinophilic material (alveolar proteinosis) with a few macrophages and desquamated type II pneumocytes and occasional neutrophils; occasionally, clumps of necrotic cells (neutrophils) are also present. Some bronchi and bronchioles are filled with a mucopurulent exudate that sometimes extends into adjacent alveoli and/or submucosal glands; their propria-submucosa is infiltrated by lymphocytes and plasma cells and there is mild to moderate BALT hyperplasia.
Contributor's Morphologic Diagnosis:
1. Lobular, moderate to marked, extensive lymphoplasmacytic interstitial and proliferative pneumonia with alveolar proteinosis, consistent with SRLV pneumonia (caprine arthritis-encephalitis/CAE).
2. Marked, subacute mucopurulent bronchitis and bronchiolitis.
Note: the samples were originally submitted as ovine tissues (ewe), but based on the typical CAE lesions and small size of red blood cells (goats have the smallest RBCs of domestic animals) we called the abattoir which confirmed the mistake.
Contributor's Comment:
Histopathological observations and IHC results are consistent with small ruminant lentivirus (SRLV) pneumonia in goats. Diseases caused by SRLV are known as caprine arthritis-encephalitis (CAE) in goats and maedi-visna (MV) in sheep; MV is also known as ovine progressive pneumonia (OPP) in the USA. The mucopurulent bronchitis and bronchiolitis are likely due to a secondary bacterial infection, although routine aerobic culture did not yield any significant bacteria (contaminants).
Lentiviruses are a genus (Lentivirus) of non-oncogenic viruses in the Retroviridae family (single-stranded RNA). This genus includes immunodeficiency viruses (HIV, SIV, FIV and BIV), the Equine infectious anemia virus (EIAV in Equidae à hematological, neurological and immunological) and small ruminant lentiviruses (SRLV).6 Lentiviruses share many similarities with other retroviruses. Their proviral RNA genome includes three structural genes (gag, pol and env) and long terminal repeats (LTRs) at each end. Two proteins encoded by the pol gene are the enzymes reverse transcriptase, which allows transcription of viral RNA into double-stranded DNA, and integrase which enables its integration into the host cells' genome.2,3,6 All lentiviruses infect and replicate in histiocytic cells; HIV, SIV, and BIV are also highly lymphotropic, in contrast to SRLV, EIAV, and BIV (the latter is only mildly immunosuppressive).2,6 Although often referred to as caprine arthritis-encephalitis virus (CAEV) in goats and maedi-visna virus (MVV) in sheep, these viruses are now considered a spectrum of SRLV variants with some detected only in sheep (classical MMV strains), some only in goats (classical CAEV strains) and some in both species; furthermore, there is now also evidence for potential dual infection.2,3,6 SRLV has also been demonstrated (PCR) in wild ibexes' (Capra ibex) monocytes, experimentally-infected Mouflon-domestic sheep hybrids, and naturally-infected Rocky Mountain goats (Oreamnos americanus) with typical CAEV lesions. This is unusual for lentiviruses which are, with a few exceptions (e.g. SIV in human and non-human primates; FIV in multiple felid species), species-specific.2
Caprine arthritis-encephalitis (CAE) in goats (Capra aegagrus hircus) and maedi-visna (MV) in domestic sheep are similar with regard to pathogenesis, pathology and clinical signs. Infection by SRLV is mainly through ingestion of colostrum/milk; inhalation of infected nasal secretions, directly or through aerosols, is another possible mode of transmission.2,3 The main targets of SRLV are histiocytic cells (monocytes/macrophages and dendritic cells) in which they establish lifelong infection because the virus is able to evade the immune system by several mechanisms.2,3,6 Among others, lentiviruses (and retroviruses in general) tend to mutate at a relatively high rate due to the error-prone reverse transcription mechanism, thus creating several variants within a cell (quasispecies); this is followed by recombination, thus creating relatively high genetic variability in viruses within an individual animal.2,6 Also, there are relatively low levels of circulating SRLV-infected cells (and free virus is rare) and the virus remains latent in monocytes, thus allowing widespread dissemination mainly to CNS, lung, mammary gland and joints.2 When monocytes become macrophages in tissues, viral transcription and protein production increases to high levels and cytokines are produced; this leads to a chronic immune-mediated reaction which involves CD4+ and CD8+ T cells, B cells/plasma cells and macrophages.2,6 SRLV also infects epithelial cells of the mammary gland and thus, as mentioned above, colostrum/milk is an important source of virus.2
Both CAE and MV cause encephalomyelitis, pneumonia, arthritis and mastitis that can occur singly or in different combinations in susceptible hosts.3,6,7 The pathologic picture depends on viral and host factors (e.g. certain breeds of sheep are more susceptible).2,3,6 They are progressive wasting diseases mainly seen in adult animals, with the exception of the neurological form of CAE which is mostly seen in 2-4 month-old goat kids; the respiratory and neurological forms are invariably fatal. Arthritis and encephalomyelitis are the most frequent pathological manifestations of CAE, while they are uncommon to rare in MV.3 Pneumonia is the most common manifestation in MV, but is relatively uncommon in CAE; maedi is the name of the respiratory form of MV.3 Mastitis may be a more frequent occurrence than generally reported in MV as a study in the province of Quebec found more mammary than pulmonary lesions in MV-seropositive sheep.1 In MV, the lungs fail to collapse when the thorax is opened, and they are diffusely pale gray to tan with a rubbery to firm texture (interstitial pneumonia). Gross lesions are similar in CAE, but they vary from patchy to diffuse.3,8 Three inflammatory patterns have been described in the CNS, lung and mammary gland of MV-affected sheep: lymphocytic (mainly CD8+ T cells), histiocytic (mainly macrophages and B cells) and mixed;4 we did not find a similar study for CAE. Pulmonary lesions in CAEV, MV and other lentiviral diseases share a common pattern of lymphocytic interstitial pneumonia (LIP). In maedi, but not in CAE, lesions are also characterized mainly by lymphoid follicular formation and smooth muscle hypertrophy; mild interstitial fibrosis is also described. The alveolar proteinosis is typical of CAE; it has been suggested this phenomenon may be more host-related than disease-related.3,8 Ultrastructurally, the intra-alveolar proteinaceous material consists of myelin figures, consistent with surfactant.8 Perivascular and peribronchiolar lymphoid infiltration with follicular formation has been described.8
Contributing Institution:
Faculty of veterinary medicine, Université de Montréal. http://fmv.umontreal.ca/faculte/departements/pathologie-et-microbiologie
JPC Diagnosis:
1. Lung: Pneumonia, interstitial, lobular, lymphohistiocytic, moderate with abundant proteinaceous alveolar edema and type II pneumocyte hyperplasia.
2. Lung: Peribronchiolar and perivascular lymphoid hyperplasia and bronchiolar epithelial hyperplasia.
3. Lung: Bronchopneumonia, suppurative, focally extensive, mild to moderate.
JPC Comment:
The contributor provides an outstanding review of the pathogenesis and various clinical manifestations of small ruminant lentiviruses (SRLVs). In addition, lentiviral mastitis and SRLVs in general were recently discussed during WSC 14, case 1.
As noted by the contributor, SRLVs were historically
believed to be species specific with maedi-visna virus infecting sheep while the
aptly named caprine arthritis and encephalitis virus (CAEV) infected goats.9
SRLVs have since been
divided into five genetically diverse genotypes (A-E), with most genotypes and
associated subtypes reported to infect both sheep and goats. However,
exceptions include the E genotype, which has only been reported in sheep, while
six A subtypes have only been reported in goats. While it is possible these
variants are species specific, it is also possible interspecies transmission has
not yet occurred or simply have not yet been detected.5
SRLVs have been recognized throughout the world for decades, with maedi first being described in South Africa in 1915. Ovine progressive pneumonia virus (OPPV) was identified less than decade later in Montana in 1923, with the affected sheep being described as "lungers" as they tended to lag behind the flock. In contrast, goats infected with SRLV were initially believed to be affected by a hereditary disease due to a perceived relationship between the clinical disease and certain family lines. The virus now known as CAEV was first identified as a cause of leukoencephalitis in the in 1974; researchers from the same laboratory later isolated the same virus from goats with chronic arthritis. Historically, SRLVs had a worldwide distribution. However, several countries are now SRLV free, including Iceland, New Zealand, and Australia.9
For reasons previously discussed by the contributor, SRLVs are associated with lifelong infection. However, only approximately 30% of infected animals develop clinical illness. The remaining animals with subclinical infections are particularly problematic as they serve as carriers, introducing the disease to offspring as well as naïve herds and flocks. In addition, subclinical infections result in significant economic loss that is not often readily apparent on an individual basis unless metrics are closely monitored. Examples of such losses include reductions in market weight of lambs reared by infected ewes (3.86kg) compared to those of unaffected ewes and seropositive goats have been associated with significantly lower length of lactation, milk yield, milk fat, and lactose levels.9
Interestingly, many producers are unaware of the risk posed by SRLVs. A 2011 study conducted by the US Department of Agriculture on operations representing over 70% of US farms with ewes and over 85% of the total ewe inventory found nearly half (46.5%) of producers were not familiar with ovine progressive pneumonia virus (i.e. SRLV). Of the 53.5% of producers aware of SRLV, over 72% did not know their current status and only 16.2% had a flock health management program implemented for the control or eradication of SRLV. Although this study did not assess seroprevalence, another 2011 study conducted by the USDA in Wyoming assessed sera from 1415 sheep and 54 flocks of various size ranges and grazing types and found 18% of sheep and 47.5% of flocks were seropositive for OPPV.9
As demonstrated by this case, characteristic features of caprine SRLV pneumonia include dense eosinophilic alveolar fluid with foamy alveolar macrophages, type II pneumocyte hyperplasia, and expansion of alveolar septa by lymphocytes and fibrosis. In contrast, type II pneumocyte hyperplasia is not a prominent feature of maedi, which is histologically characterized by lymphoplasmacytic infiltrates expanding septa, perivascular tissue, and airways which often form characteristic lymphoid follicles.3
Participants were in agreement with the contributor in regard to the likelihood of a secondary bacterial component in this case. The moderator strongly suspected this goat had concurrent bronchopneumonia due to Mycoplasma spp. such as M. ovipneumoniae due to the presence of characteristic peribronchiolar and perivascular lymphoid hyperplasia, bronchiolar epithelial hyperplasia, and negative aerobic bacterial culture. However, given both SRLV and Mycoplasma spp. cause lymphohistiocytic interstitial pneumonia it is not possible to definitively ascertain the cause of the secondary bronchopneumonia without additional diagnostics such as PCR.
References:
1. Arsenault J, Girard C, Dubreuil P, et al. Prevalence of and carcass condemnation from maedi visna, paratuberculosis and caseous lymphadenitis in culled sheep from Quebec, Canada. Prev Vet Med. 2003;59(1-2):67-81.
2. Blacklaws BA. Small ruminant lentiviruses: immunopathogenesis of visna-maedi and caprine arthritis and encephalitis virus. Comp Immunol Microbiol Infect Dis. 2012;35(3):259-269.
3. Caswell JL, Williams KJ. Respiratory system. In: Jubb, Kennedy and Palmer's Pathology of domestic animals, vol. 2. Sixth Edition. Elsevier. St.Louis, Mo, 2016:465-591.
4. Gayo E, Polledo L, Balseiro A, et al. Inflammatory Lesion Patterns in Target Organs of Visna/Maedi in Sheep and their Significance in the Pathogenesis and Diagnosis of the Infection. J Comp Pathol. 2018;159:49-56.
5. Highland MA. Small Ruminant Lentiviruses: Strain Variation, Viral Tropism, and Host Genetics Influence Pathogenesis. Vet Pathol. 2017;54(3):353-354.
6. Minguijón E, Reina R, Pérez M, et al. Small ruminant lentivirus infections and diseases. Vet Microbiol. 2015;181(1-2):75-89.
7. Patton KM, Bildfell RJ, Anderson ML, Cebra CK, Valentine BA. Fatal Caprine arthritis encephalitis virus-like infection in 4 Rocky Mountain goats (Oreamnos americanus). J Vet Diagn Invest. 2012;24(2):392-396.
8. Sims LD, Hale CJ, McCormick BM. Progressive interstitial pneumonia in goats. Aust Vet J. 1983;60(12):368-371.
9. Wolf C. Update on Small Ruminant Lentiviruses. Vet Clin North Am Food Anim Pract. 2021;37(1):199-208.