CASE I: N-521/18 (JPC 4137931)
Signalment:
3 year-old. Female. Rasa Aragonesa. Ovine (Ovis orientalis aries)
History:
The animal was presented with agalactia to the clinical service for ruminants (Servicio Clínico de Rumiantes ? SCRUM) of the University of Zaragoza two months after parturition. At clinical examination, the animal showed induration of the mammary gland and mild diffuse increase of respiratory sounds.
Gross Pathology:
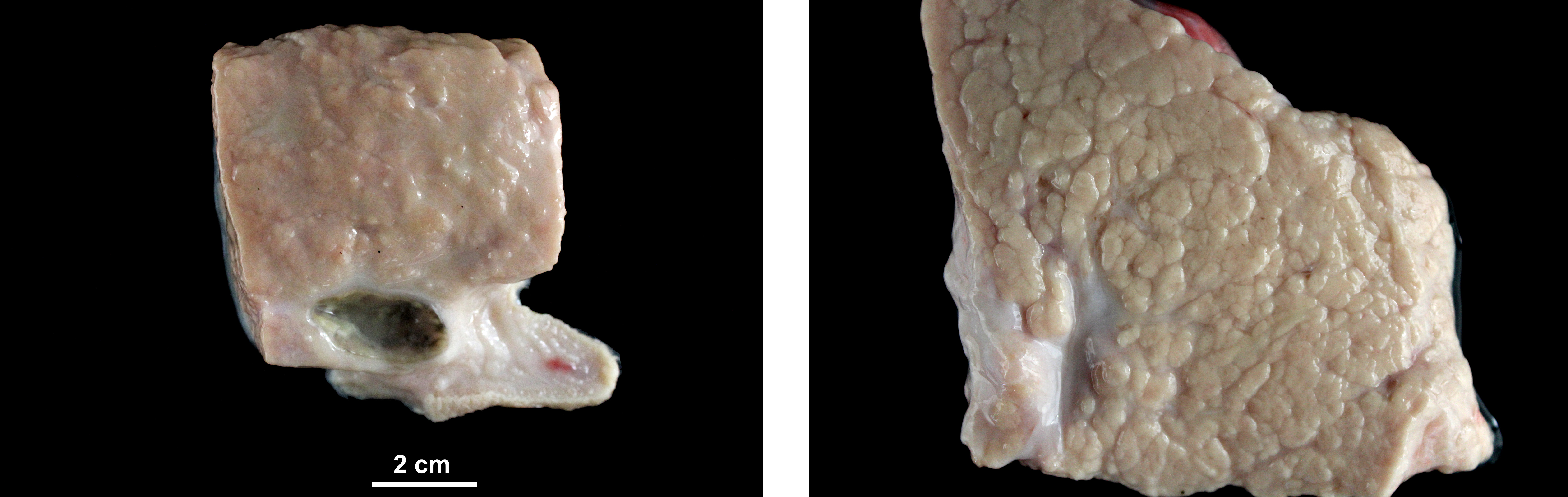
At necropsy, the sheep showed body score of 3/5. Mammary gland presented mild diffuse enlargement and severe increase in firmness. Lungs were increased in size and weight and showed greyish discoloration. Mediastinal and tracheobronchial lymph nodes were enlarged.
Laboratory Results:
The animal was seropositive to small ruminant lentivirus (SRLV, Elitest MVV/CAEV, Hyphen Biomed).
Microscopic Description:
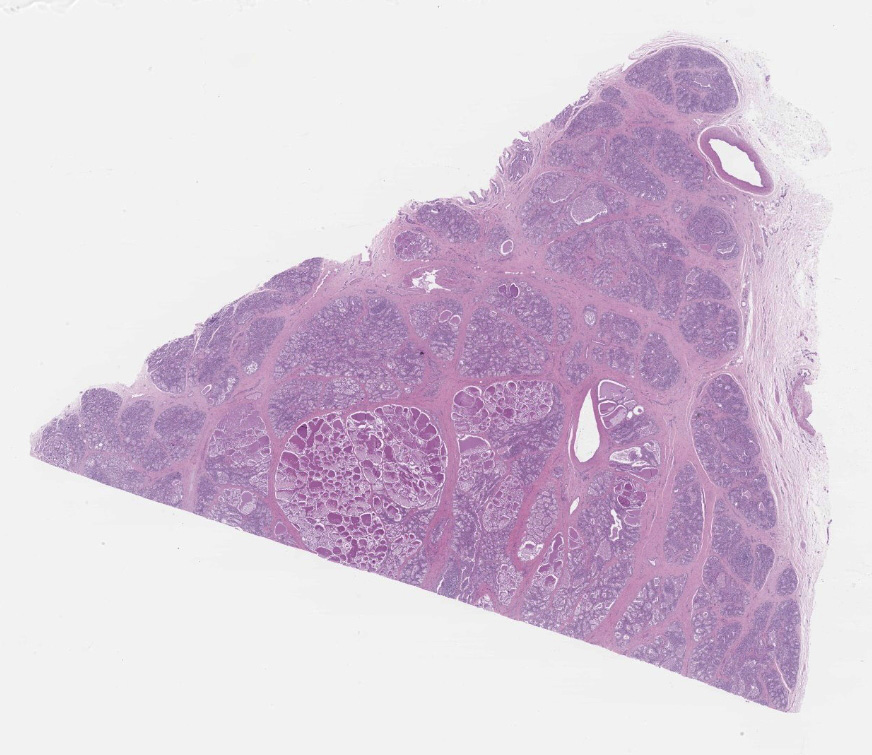
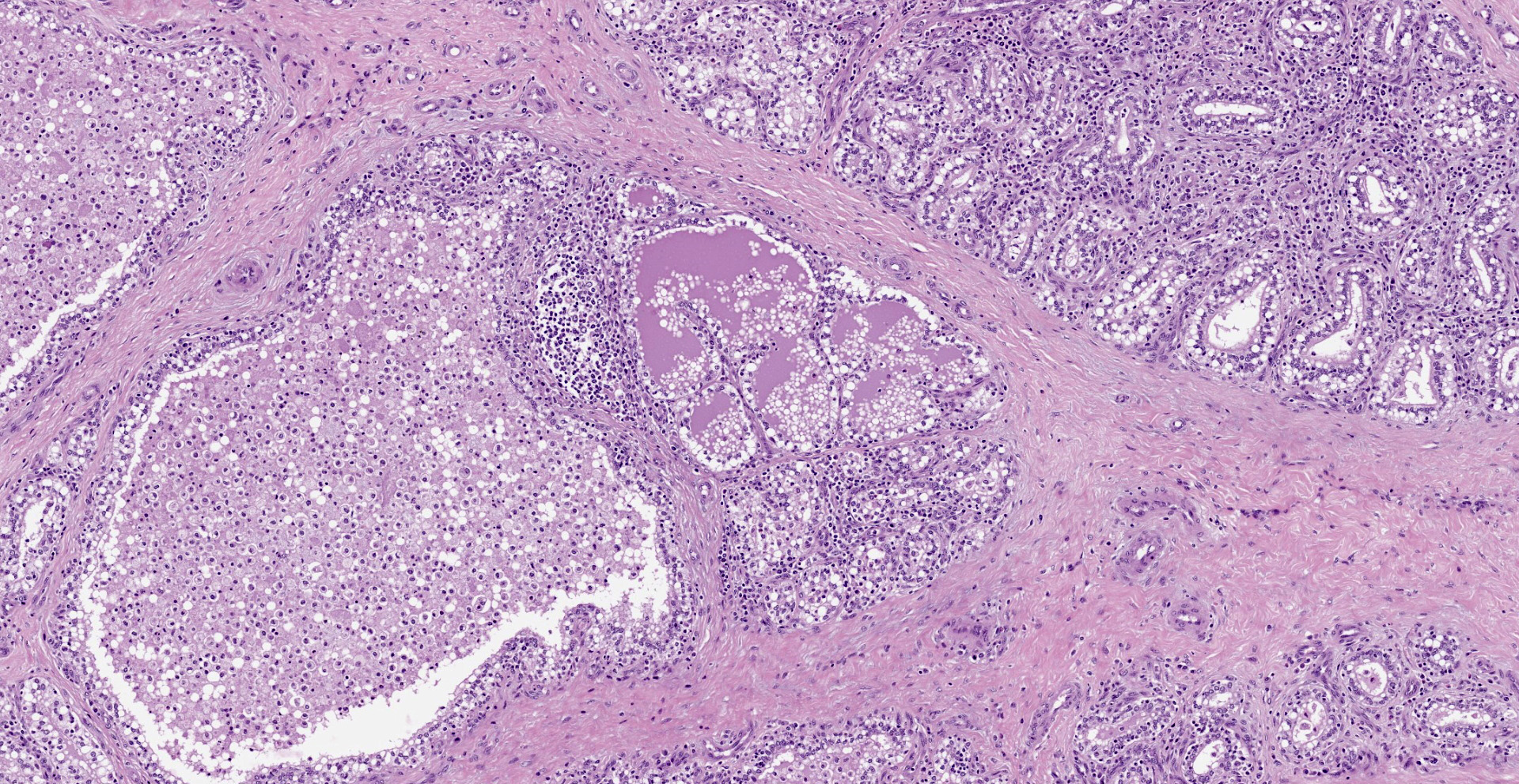
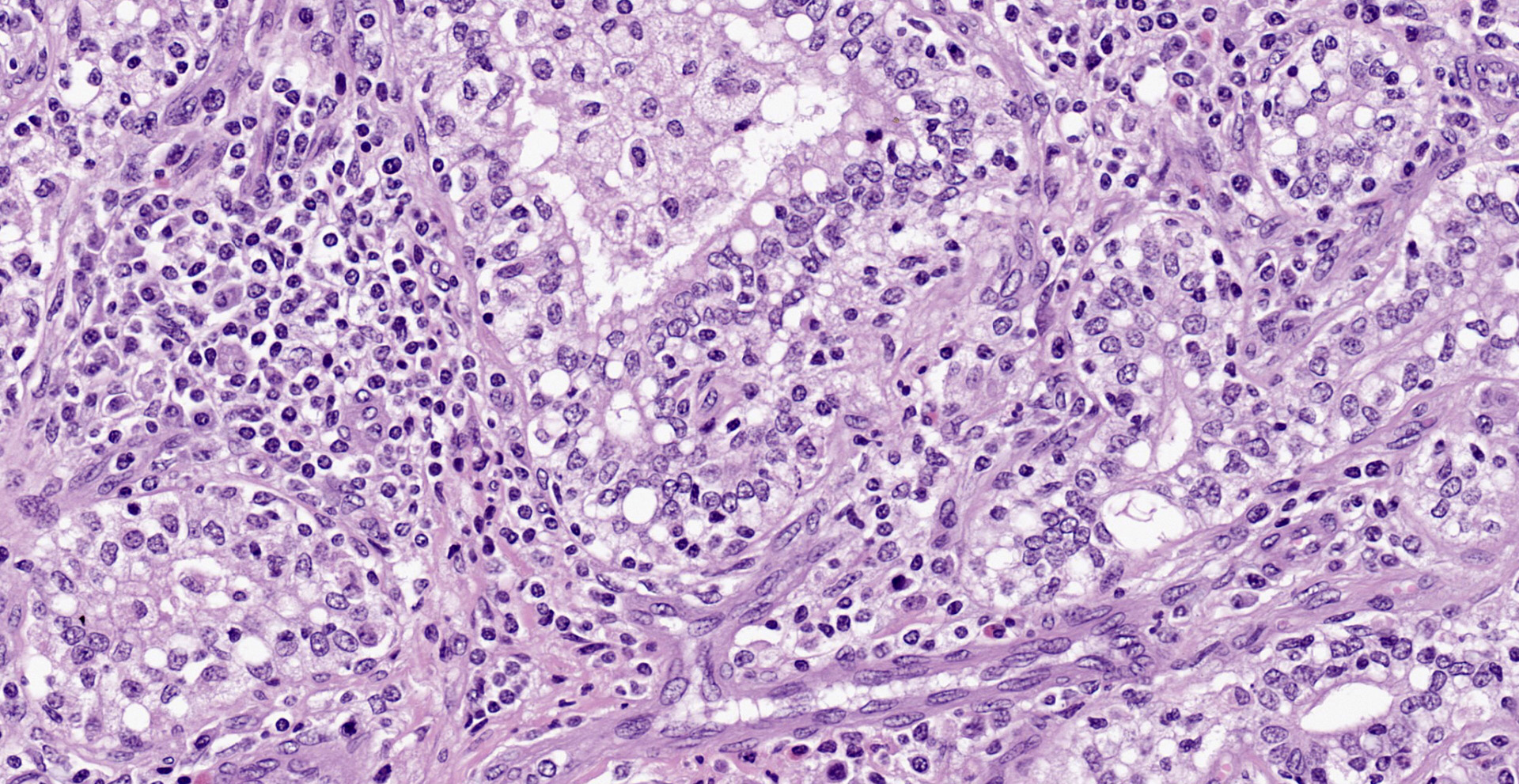
Diffusely, affecting up to the 90% of the section, there are inflammatory and degener-ative changes in the acini, inter- and intralobular ducts and intersititium that efface the normal structure. Multifocally, mammary interstitium is infiltrated by abundant lymphocytes and less plasma cells, with occasionally formation of large follicular structures (not in all slides). There are lesser numbers of histiocytes and rare neutrophils. Multifocally, acini are reduced in size (atrophy) and show attenuated, disorganized, swollen and/or vacuolated epithelium (degeneration), with occasional acinar collapse with pyknosis and karyorrhexis (necrosis). Lumina of acini are rarely filled with an eosinophilic proteinaceous fluid, with occasional clear lipidic droplets and abundant foamy macrophages with occasional multinucleated giant cells. Diffusely, inter- and intralobular septae are prominent and infiltrated by mild numbers of the previously-described inflammatory cells, generally in a perivascular location as well as moderate numbers of plump fibroblast intermingled with immature collagen fibers (intralobular fibrosis).
Contributor's Morphologic Diagnoses:
Mammary gland: Mastitis, interstitial, lymphoplasmacytic, diffuse, severe, with lymphoid follicle formation, acinar atrophy and degeneration and mild intralobular fibrosis.
Contributor's Comment:
This is the mammary form of small ruminant lentiviruses (SRLV). This chronic disease is caused by a group of non-oncogenic exogenous retroviruses of the genus lentivirus that target immune cells, mainly macrophages.
The SRLVs are a group of highly related single stranded RNA viruses with high mutational potential that affect sheep and goats.11 These viruses induce chronic inflammation that usually remains subclinical. When it becomes clinical, the disease can express mainly in four different locations: lung, joints (mainly carpus), central nervous system and mammary gland.7 The expression of each form as well as the severity of the lesion depends on the host immune response and the viral factors.3,9
Mammary form is difficult to detect and mainly consist of mammary gland induration, progressive bilateral atrophy and milk production loss that leads to deficient lamb growth.6 Both mammary glands are usually symmetrically affected. Microscopic lesions are characterized by abundant lymphocytes and plasma cells that infiltrate the interstitial and periductal connective tissue and even form lymphoid nodules. In advanced lesions degeneration and loss of acinar and ductular epithelium may happen as a consequence of inflammation rather than direct virus effect. Strikingly, interstitial fibrosis is a controversial histologic finding of lentiviral mastitis.2,7,13 As far as contributors are concerned, interlobular fibrosis is hard to determine because of microscopic appearance of the mammary septa is highly variable according to the physiological condition of the animal. However, intralobular fibrosis is more challenging to interpret and authors question if the moderate intralobular fibrosis present in this case has been induced by the viral infection or is the reflection of the acinar atrophy and collapse. In goats affected by the SRLV, mastitis is not commonly detected.
Respiratory form is the most common presentation of the disease in sheep and consist of progressive pneumonia with marked dyspnea and weight loss.1 Lungs are characterized by showing a rubbery firm texture and lack of collapse when thorax is opened. Indeed, this animal presented with a diffuse interstitial pneumonia with BALT hyperplasia (not submitted). Neurologic form is characterized by demyelinating leukoencephalomyelitis that leads to ataxia and profound weight loss.10 This form progresses faster than the respiratory one and usually occurs in adult sheep and goat kids (2-4 months). Articular form is characterized by arthritis affecting mainly the carpal joints.8
The main route of transmission is aerosol, particularly under intensive housing. Colostrum transmission plays also an important role.7 The virus infects a variety of cell types (mammary epithelium, fibroblast, endothelial cells, monocytes, choroid plexus) but its replication mainly occurs in mature macrophages.11 Lentiviral infected macrophages produce cytokines that recruit and activate other leukocytes but also enhance lentiviral replication. Indeed, clinical signs and histopathological lesions are the result of the inflammatory process instead direct viral damage to the organ. There is neither treatment nor commercial vaccines for the disease, what makes challenging the immunization of the ovine population against the virus.12
The main differential diagnosis for this kind of mastitis in the Mediterranean countries is contagious agalactia caused by Mycoplasma agalactiae, a septicemic pathogen with high mortality rates that can cause also polyarthritis and keratoconjunctivitis. Staphylococcus aureus, Mannheimia haemolytica and Corynebacterium pseudotuberculosis should also be considered, although unilateral involvement and suppurative and/or necrotizing mastitis should be expected with these pathogens.13
Contributing Institution:
Universidad de Zaragoza. Departamento de Patología Animal
https://patologiaanimal.unizar.es
JPC
Diagnosis:
Mammary gland: Mastitis, lymphohistiocytic and neutrophilic, multifocal, lobular, marked, chronic with fibrosis.
JPC Comment:
The contributor provides a concise review of the pathogenesis and multiple clinical manifestations associated with small ruminant lentiviruses (SRLVs), an economically significant group of viruses distributed worldwide, with notable exceptions including Iceland, New Zealand, and Australia.15
SRLVs are a group of viruses in the genus Lentivirus in the Retroviridae family that include caprine arthritis and encephalitis virus (CAEV), maedi-visna virus (MVV), and ovine progressive pneumonia virus (OPPV). Lifelong infection associated with a slow onset (lentus; Latin for "slow") of clinical signs is a key feature of SRLVs.5 These entities are aptly named given MVV is associated with chronic respiratory disease (maedi = shortness of breath) and neurologic disease (visna = wasting due to meningoencephalitis) while CAEV is classically associated with arthritis and/or encephalitis. Although these viruses were historically believed to be host specific, with CAEV infecting goats while MVV/OPPV infected sheep; however, interspecies transmission has been reported.5
SRLVs are divided into five genetically diverse genotypes (A-E). There is 25-37% nucleotide sequence variation between types as the result of common point mutations (largely due to a lack of proofreading ability of the viral transcriptase) and recombination events mediated by coinfections. As previously alluded to in regard to interspecies
transmission, most genotypes and associated subtypes have been reported in both sheep and goats. However, only the E genotype has been reported in sheep while six A subtypes have only been reported in goats. These reports may be indicative of host specificity of some subtypes and a genotype, though it is also possible interspecies transmission has not yet occurred or simply has not yet been identified.5
SRLVs can present a challenge for producers to control given its ability to undergo transmission both vertically via infected colostrum and milk as well as horizontally via secretions/excretions from the respiratory, urogenital, and digestive tracts. In addition, only 30% of SRLV infected animals develop clinical disease whereas the remaining animals may remain subclinically infected for life. Control measures involving neonates include separation of the newborn from the dam prior to suckling, feeding pasteurized colostrum, and subsequently hand/artificially rearing the animal. Strict biosecurity measures, including screening of animals prior to herd/flock entry, lifelong separation of carriers, and elimination or disinfection of shared fomites (e.g. automatic milkers), and testing programs are utilized to control transmission.5
Many producers are unaware ewes or does have developed mastitis until the affected
dam's offspring are found to be malnourished or have died. Clinically, the udders appear
bilaterally normal but are firm without pitting edema upon palpation and only a small amount of milk can be manually expressed.
One study found weaning weights of lambs reared by SRLV-infected ewes were decreased (3.86-4.95kg) when compared to those reared by SRLV-negative ewes, which correlates to significant financial loss for a producer over the ewe's lifetime.6,15 Affected animals are typically culled; however, SRLV negative offspring can be reared from SRLV positive dams as previously discussed. Although the intensive hand rearing process is a costly alternative, it provides an option to preserve valuable bloodlines.15
Testing is an essential tool utilized by producers to eliminate SRLV infected animals subsequently maintain SRLV free flocks. Commonly tested animals include weaned replacement lambs, yearlings that have not yet intermixed with mature ewes, and ewes (and rams) that are of an unknown status prior to herd entry. Serum based ELISA testing is the most common method, which is highly sensitive though false positives do occur. Upon testing positive, seropositive animals are segregated from seronegative animals and are commonly marked (e.g. bright ear tag) to prevent inadvertent intermixing. Newly purchased animals, as well as those returning from shows with contact with other small ruminants are tested upon arrival, quarantined, and tested again 60 days later prior to release. Unfortunately early infections cannot be detected using ELISA. It is therefore common to test these animals again at two to three month intervals until two to three consecutive whole-group tests have been completed. In addition to false positives, false negatives have also been reported with ELISA testing. This situation typically becomes apparent when a flock is seronegative over consecutive tests but seropositive animals are identified in future whole-flock tests. One protocol studied to overcome this phenomenon combined the use of highly sensitive ELISA testing with PCR to detect proviral SRLV sequences integrated into the animal's genome once every three months. The leader-gag sequence was selected for RT-PCR due to being highly conserved despite SRLV's high rate of mutations. PCR testing identified some infected animals prior to becoming seropositive, facilitating the study herd's return of to a SRLV negative status after three rounds of testing at two to three month intervals. Although effective, cost is a significant disadvantage of this approach.15
An additional control strategy under investigation is the use of host genetics given some genotypes/polymorphisms have been associated with SRLV resistance or low viral loads, which have been shown to correlate with decreased lesion severity. In addition, it is also likely decreased viral loads present a lower risk of transmission. One potential gene found to be associated with decreased SRLV susceptibility or lower viral load in sheep is the transmembrane 154 gene (TMEM154), which encodes a protein of an unknown function. Although selective breeding programs are a potentially effective component of an overall control strategy, purpose-bred resistance may be overcome as the result of rapid mutations associated with SRLVs.5
Any mention of MVV or CAEV would be remiss without discussion of typical clinical manifestations of neurologic disease associated with ages of the infected animals. Although older goats may also be affected with the neurologic form of CAEV, it most frequently occurs in kids between one to six months of age whereas visna affects adult sheep. Affected kids initially develop a pelvic limb paresis that progresses to paralysis of all four limbs. Visna presents as two forms in the sheep, depending on the region of the CNS affected. Lesions affecting the brain arise in the lateral ventricles and result in a head tilt and circling toward the affected side. Sheep with lesions in the spinal cord classically present with unilateral knuckling of a pelvic limb while the contralateral limb retains the ability to bear weight.15
During the conference the moderator discussed the importance of subclinical animals acting as reservoirs within herds. This was further highlighted in a recent study14 conducted in Italy evaluating subclinical mastitis which 89 macroscopically healthy sheep and goat udders were cultured and submitted for histologic evaluation. Infectious agents were identified in 64 (71.9%) cases. Coagulase negative staphylocci were the most prevalent bacteria isolated (46.2%), followed by environmental opportunistic bacterial pathogens (34.7%). 75% of infected mammary glands had co-infections and a total of 138 different bacteria were isolated. In addition, lentiviruses were detected in 39% of infected udders.14 Histologically, 45/89 udders demonstrated features of chronic non-suppurative mastitis, followed by chronic mixed mastitis (12/89), and acute suppurative mastitis (4/89). Only 28/89 udders were histologically normal. Therefore, small ruminants with macroscopically healthy udders may act as reservoirs for microbial agents within herds and flocks14, further necessitating herd health surveillance programs such as previously discussed.
References:
1. Blacklaws BA. Small ruminant lentiviruses: Immunopathogenesis of visna-maedi and caprine arthritis and encephalitis virus. Comp Immunol Microbiol Infect Dis. 2012;35(3):259?269.
2. Bolea R, Monleón E, Carrasco L et al. Maedi-visna virus infection of ovine mammary epithelial cells. Vet Res. 2006; 37: 133-144.
3. Gayo E, Polledo L, Balseiro A, et al. Inflammatory Lesion Patterns in Target Organs of Visna/Maedi in Sheep and their Significance in the Pathogenesis and Diagnosis of the Infection. J Comp Pathol. 2018;159:49?56.
4. Gayo E, Polledo L, Magalde A, et al. Characterization of minimal lesions related to the presence of visna/maedi virus in the mammary gland and milk of dairy sheep. BMC Vet Res. 2019;15:109. 1-9.
5. Highland MA. Small Ruminant Lentiviruses: Strain Variation, Viral Tropism, and Host Genetics Influence Pathogenesis. Vet Pathol. 2017;54(3):353-354.
6. Keen JE, Hungerford LL, Littledike ET, et al. Effect of ewe ovine lentivirus infection on ewe and lamb productivity. Prev Vet Med 1997;30:155?69.
7. Minguijón E, Reina R, Pérez M, et al. Small ruminant lentivirus infections and diseases. Vet Microbiol. 2015;181(1?2):75?89.
8. Pérez M, Biescas E, Reina R, et al. Small Ruminant Lentivirus?Induced Arthritis.
Vet Pathol. 2015;52(1):132?139.
9. Pinczowski P, Sanjosé L, Gimeno M, et al. Small Ruminant Lentiviruses in Sheep.
Vet Pathol. 2017;54:413?424.
10. Polledo L, González J, Benavides J, et al. Patterns of Lesion and Local Host Cellular Immune Response in Natural Cases of Ovine Maedi-Visna. J Comp Pathol. 2011;147(1):1?10.
11. Pritchard GC, McConnel I. Maedi-Visna. In: Aitken ID, editor. Diseases of sheep. Fourth Edition. Oxford (UK): Blackwell Publishing; 2007. 217?223.
12. Reina R, de Andrés D, Amorena B. Immunization against small ruminant lentiviruses. Viruses. 2013;5:1948?1963.
13. Schlafer D, Foster R. Female Genital System. In: Maxie GM, ed. Pathology of Domestic Animals. Volume 3. 2016:358?465.
14. Spuria L, Biasibetti E, Bisanzio D, et al. Microbial agents in macroscopically healthy mammary gland tissues of small ruminants. PeerJ. 2017;5:e3994. Published 2017 Nov 13.
15. Wolf C. Update on Small Ruminant Lentiviruses. Vet Clin North Am Food Anim Pract. 2021;37(1):199-208.