WSC 23-2 Conference 9, Case 4:
Signalment:
10-month-old, male Dorper sheep, ovine (Ovis aries)
History:
The farm has a Dorper sheep herd comprising 1500 animals. Four sheep showed clinical respiratory signs characterized by cough and tachypnea. They were administered antibiotics and nonsteroidal anti-inflammatory drugs, but no clinical improvement was noted. Fifteen animals died within 10 days. One sheep was euthanized owing to poor quality of life and underwent necropsy.
Gross Pathology:
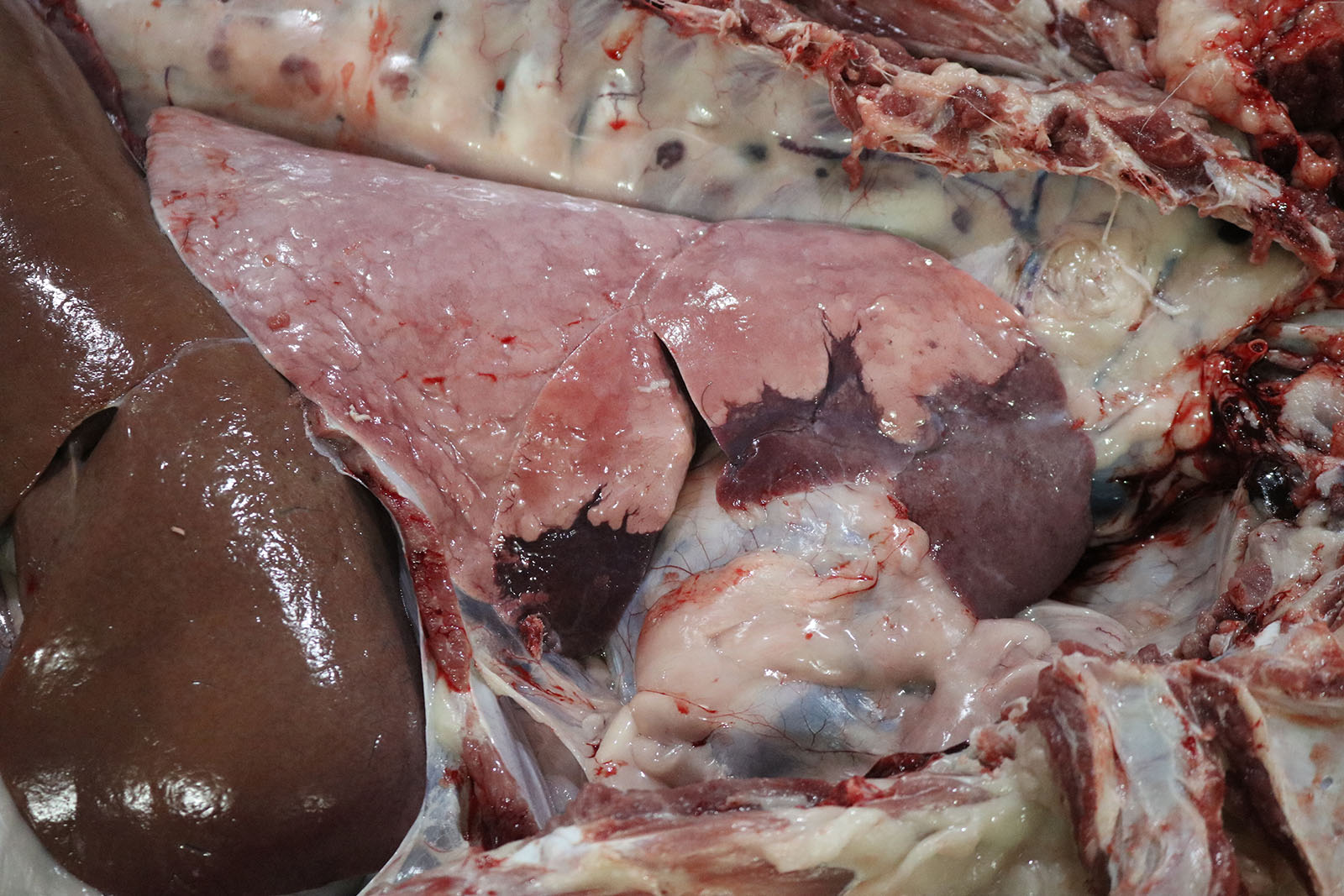
Lung: Gross examination revealed bilaterally consolidated areas that were focally extensive, dark red, and firm on palpation, affecting the cranioventral region of the right (40%) and left (10%) lobes. On cut surface, the parenchyma was light brown to dark red and consolidated. A large amount of translucent seromucous fluid was seen at the opening of the bronchi.
Laboratory Results:
Bacteriology: Pasteurella multocida and Mannheimia haemolytica PCR were negative (lung).
Virology: PCR of the lung tissue was positive for Mycoplasma spp. and lentivirus of small ruminants, which were confirmed as Mycoplasma ovipneumoniae and Maedi visna using genetic sequencing.
Microscopic Description:
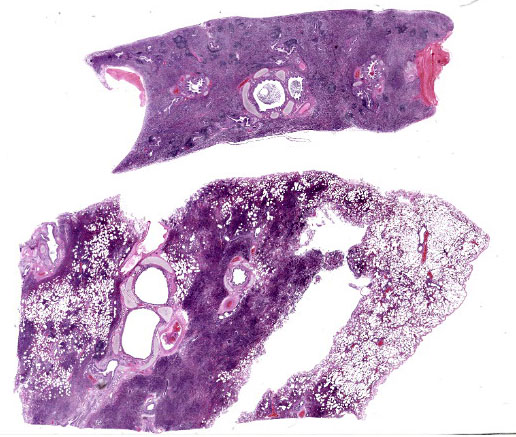
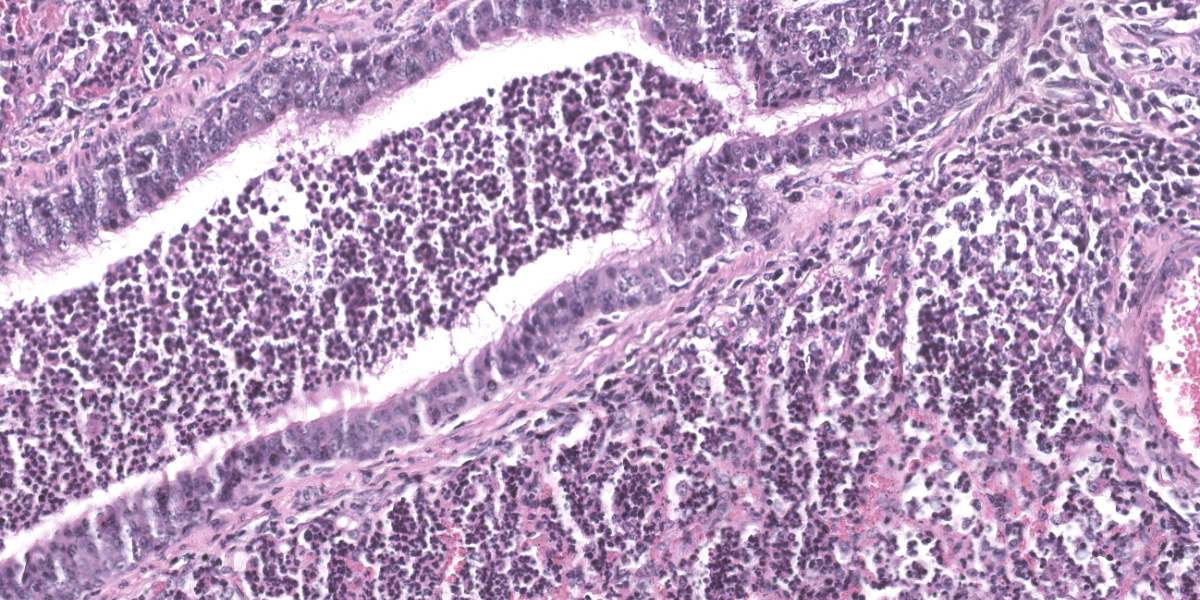
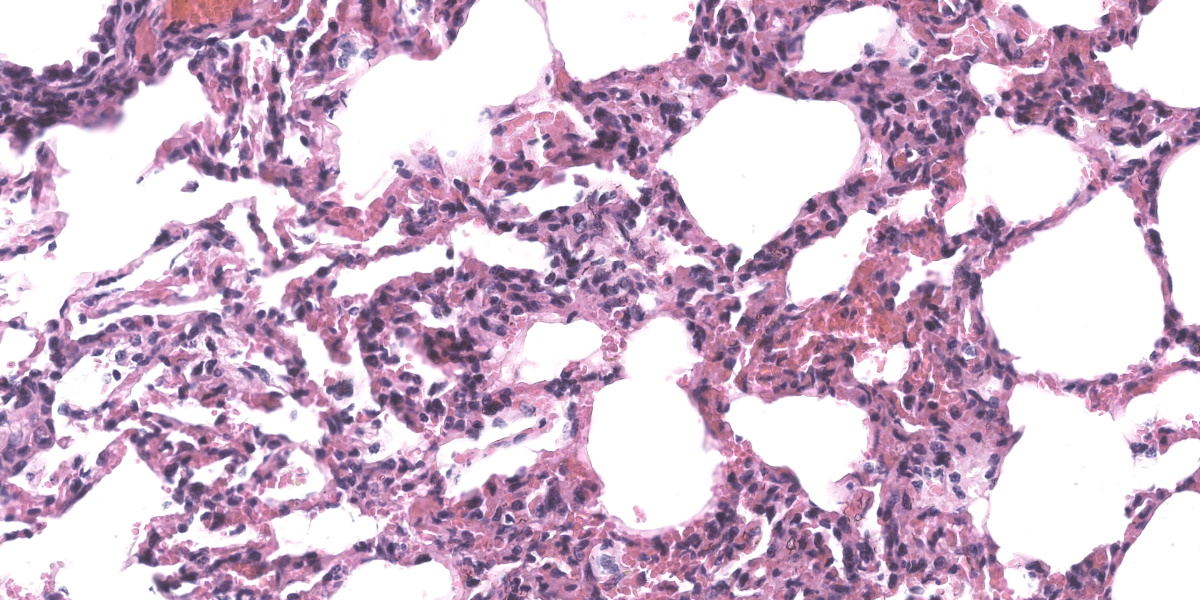
Lung: Alveolar lumina are variably filled by large numbers of neutrophils and macrophages admixed with mild edema affecting 30-40% of the parenchyma within submitted sections. Multifocally, moderate numbers of affected alveoli are lined with plump and cuboidal epithelial cells (type II pneumocyte hyperplasia). Multifocally, the lumens of the bronchi and bronchioles are also filled with variable numbers of neutrophils admixed with fewer macrophages. The submucosa of the bronchi and bronchioles, as well as the adventitia of the blood vessels, are frequently expanded by cuffs of lymphocytes and plasma cells arranged in follicular aggregates.
Contributor’s Morphologic Diagnosis:
Lung: Bronchopneumonia, suppurative and histiocytic, chronic-active, diffuse, severe, with bronchial epithelial and type II pneumocyte hyperplasia and peribronchiolar lymphofollicular proliferation.
Contributor’s Comment:
Maedi-visna (MV), also known as ovine progressive pneumonia (OPP), is an incurable viral disease in sheep with a prolonged incubation period that develops into a life-long infection.3 This disease is caused by non-oncogenic exogenous retroviruses, namely maedi-visna virus (MVV) and caprine arthritis-encephalitis virus (CAEV), both of which belong to a subgroup of viruses known as small ruminant lentiviruses (SRLVs).10 For years, lentiviruses isolated in ovines were considered MVV and ones in caprines were considered CAEV, and the two were considered to be species-specific; however, phylogenetic analyses and findings of cross-infection have demonstrated differences in genotypes and lentiviral subtypes that can infect both goats and sheep.1,5-7,12,14,16,17
SRLVs exhibit tropism for the mononuclear-phagocyte system and induce slow, chronic, and persistent inflammation mainly in four target organs (the lung, joints, nervous system, and mammary gland), resulting in different clinical phenotypes (i.e., pulmonary, articular, nervous, and mammary, respectively). Interestingly, the occurrence of each clinical form and lesion severity depends on viral factors, as well as the host immune response.2,4,9,11 In the present case, the lung lesions were typical of SRLVs, characterized by lymphoplasmacytic infiltration in septa with type II pneumocyte hyperplasia and infiltration of lymphocytes in the bronchi and bronchioles, and eosinophilic exudates in alveoli.3,17
The farm where the outbreak occurred had a system of extensive breeding without control measures. Ewes and breeding males were acquired from another Brazilian state, Bahia, where seroprevalence studies have demonstrated the presence of SRLV.15 Thus, it is probable that the purchase of these animals introduced the disease into the herd via asymptomatic animals.
In the present case, M. ovipneumoniae was detected by PCR from pulmonary tissue. M. pneumoniae is a respiratory bacterium commonly detected in both healthy and diseased lambs.8 Although speculation, it is possible that Maedi, a retrovirus, may cause immunosuppression, thereby contributing to the establishment of subsequent ovipneumoniae colonization.
A definitive diagnosis of maedi was made based on the characteristic lesions identified grossly and histopathologically, along with a supportive clinical history of the disease. The diagnosis was confirmed by molecular techniques.
Contributing Institution:
Laboratório de Patologia Veterinária
Faculdade de Medicina Veterinária
Universidade Federal de Mato Grosso
Brazil
JPC Diagnosis:
- Lung: Bronchopneumonia, neutrophilic, diffuse, moderate, with bronchiolar epithelial hyperplasia, alveolar collapse, and peribronchiolar and perivascular lymphoid hyperplasia.
- Lung: Pneumonia, interstitial, lymphohistiocytic, multifocal, mild.
JPC Comment:
Maedi-Visna virus (MVV) was first described in Iceland in 1954 by Bjorn Sigurdsson, who made history three years later by isolating MVV and thereby becoming the first scientist to isolate a lentivirus. Maedi-visna and Iceland have history that began in 1933 when the country imported several Karakul sheep tasked with improving the native Icelandic sheep breed. Unfortunately, Icelanders had the wool pulled over their eyes as the apparently healthy Karakul managed to introduce ovine pulmonary adenomatosis, pseudotuberculosis, and MVV to the native herds.18 The long incubation period characteristic of MVV delayed the first clinically apparent epidemic until six years after importation of the sheep, allowing the virus to spread throughout the country undetected and unimpeded for years.18
What followed the discovery of MVV was a concerted effort to understand, contain, and eradicate the disease. The disease name originates in the Icelandic words for dyspnea (maedi) and wasting (visna), and the virus was officially designated Maedi-Visna Virus to honor the outstanding efforts of Icelandic scientists.18 Though it persists throughout most countries in the world today, the disease was eradicated from the Icelandic islands during the 1960s through mass slaughter of sheep on affected farms.
MVV is, in many ways, a typical lentivirus, though it differs from its genus-mates in that it typically does not cause immunosuppression. Lentiviruses are a genus of retroviruses characterized by causing slowly progressing disease. Members of the Retroviridae family are unique in possessing a reverse transcriptase that transcribes the linear, positive-sense single stranded RNA genome into double stranded DNA.13 This reverse transcriptase is transcribed by one of the three genes that make up the minimum armamentarium of any self-respecting retrovirus: gag, pol, and env. The gag (group-specific antigen) gene encodes structural proteins, the pol (polymerase) gene encodes the reverse transcriptase and integrase enzymes, and env (envelope) encodes the major envelope glycoprotein.13
Retroviruses gain entry to host cells through interactions with the envelope glycoprotein and a specific cell receptor that varies depending on the individual virus. Once inside the cell, reverse transcriptase synthesizes double stranded DNA copies of the viral genome in the host which move to the nucleus and are integrated randomly into the host genome via viral integrase.13 Viral DNA is then transcribed using host cell replication machinery, and mature virions are assembled in the host cytoplasm and acquire an envelope as they bud from the host cell membrane.
Infection with MVV is frequently subclinical, and although virus is widely distributed throughout affected animals, MVV is transmitted mainly in pulmonary exudates, colostrum, and milk.13 The wide distribution of affected tissues is due to a unique “Trojan horse” pathogenic mechanism where provirus integrated into the genome of monocytes and their precursors is activated only when monocytes develop into macrophages.13 This restricted viral replication in monocytes permits MVV to transit surreptitiously through the body with minimal immune stimulation.
In conference, Dr. Williams emphasized how much information can be gleaned from subgross examination of this particular slide. The most striking feature is the complete lack of air in the majority of the submitted sections. The affected area is sharply demarcated on the gross aspect (Fig. 4-1) and represents complete alveolar collapse, and Dr. Williams emphasized that these changes should prompt participants to consider small airway disease. Subsequent examination of the bronchioles and surrounding vessels revealed bronchial epithelial hyperplasia and robust smooth muscle within arteriole walls. The arteriolar medial hypertrophy is likely due to the lung’s paradoxical response to hypoxia; if the alveoli are not being properly ventilated, endothelin-mediated vasoconstriction attempts to match perfusion with ventilation and shunts the blood away from the ineffectual alveoli. With chronicity, this can result in the arteriolar medial hypertrophy seen throughout this section.
There was robust discussion about ascribing particular histologic lesions to either MVV or Mycoplasma ovipneumonia in these particular sections. Generally, MVV should cause BALT hyperplasia and lymphohistiocytic interstitial pneumonia, while M. ovipneumonia should cause airway epithelial hypertrophy and an exudative neutrophilic bronchitis and bronchiolitis. Participants believed that the majority of the histologic lesions were likely attributable to Mycoplasma ovipneumonia, as the section was dominated by airway disease and BALT hyperplasia. In less affected areas of the lung, where alveoli were inflated and septa could be properly evaluated, a mild interstitial lymphohistiocytic pneumonia was present, though this was deemed a minor lesion in the evaluated section.
Participants also noted the history, which describes a quickly moving epidemic, and the young age of the animal. Both factors are more consistent with Mycoplasma ovipneumonia disease since MVV typically only causes clinical signs in animals many years post-infection. Participants vigorously discussed combining all histologic changes into a single morph; however, a reluctant, sheepish consensus was eventually reached to split the lesions into multiple morphologic diagnoses. The first diagnosis emphasizes participants’ assessment that the major histologic lesions were attributable to Mycoplasma ovipneumonia, while the second diagnosis emphasizes the mild interstitial disease that is potentially attributable to MVV.
References:
- Angelopoulou K, Karanikolaou K, Papan-astasopoulou First partial characterization of small ruminant lentiviruses from Greece. Vet Microbiol. 2005; 109(1-2):1-9.
- Blacklaws BA. Small ruminant lentiviruses: Immunopathogenesis of visna-maedi and caprine arthritis and encephalitis virus. Comp Immunol Microbiol Infect Dis. 2012;35(3):259-269.
- De Boer GF. Zwoegerziekte virus, the causative agent for progressive interstitial pneumonia (maedi) and meningo-leucoencephalitis (visna) in sheep. Res Vet Sci. 1975;18:15–25.
- Gayo E, Polledo L, Balseiro A, et al. Inflammatory Lesion Patterns in Target Organs of Visna/Maedi in Sheep and their Significance in the Pathogenesis and Diagnosis of the Infection. J Comp Pathol. 2018;159:49-56.
- Gjerset B, Rimstad E, Teige J, et Impact of natural sheep-goat transmission on detection and control of small ruminant lentivirus group C infections. Vet Microbiol. 2009;135(3-4):231-238.
- Kuhar U, Barlic-Maganja D, Grom J. Phylogenetic analysis of small ruminant lentiviruses detected in Slovenia.Vet Microbiol. 2003;162(1):201-206.
- Leroux C, Cruz JCM, Mornex SRLVs: A genetic continuum of lentiviral species in sheep and goats with cumula-tive evidence of cross species transmission. Curr HIV Res. 2010;8(1):94-100.
- Manlove K, Branan M, Baker K, et al. Risk Factors and Productivity Losses Associated with Mycoplasma Ovipneumoniae Infection in United States Domestic Sheep Operations. Prev Vet Med. 2019;168:30-38.
- Minguijo?n E, Reina R, Pe?rez M, et al. Small ruminant lentivirus infections and diseases. Vet Microbiol. 2015;181(1-2):75-89.
- Pa?lsson PA. Maedi-Visna. History and Clinical Description. In: Pe?tursson G, Hoff-Jørgensen R, eds. Maedi-Visna and Related Diseases: Developments in Veterinary Virology. Kluwer Academic Publishers;1990:3-74.
- Pinczowski P, Sanjose? L, Gimeno M, et Small Ruminant Lentiviruses in Sheep: Pathology and Tropism of 2 Strains Using the Bone Marrow Route. Vet Pathol. 2017;54(3):4131424.
- Pisoni G, Bertoni G, Manarolla G, et al. Genetic analysis of small ruminant lentiviruses following lactogenic transmission. Virology. 2010;407(1):91-99.
- Quinn PJ, Markey BK, Leonard FC, et al. Veterinary Microbiology and Microbial Disease. 2nd ed. Wiley-Blackwell;2011:619-633.
- Reina R, Mora MI, Glaria I, et Molecular characterization and phylogenetic study of Maedi Visna and Caprine Ar- thritis Encephalitis viral sequences in sheep and goats from Spain. Virus Res. 2006;121(2):189-198.
- Sampaio TS, Costa JN, Martinez PM, et Estudo sorológico da Maedi-Visna pelo método da imunodifusão em gel de ágar em rebanhos ovinos de Juazeiro, Bahia, Brasil. Rev Bras Saúde Prod. An. 2007;8:276-282.
- Shah CA, Bo?ni J, Huder JB, et al. Phylogenetic analysis and reclassification of caprine and ovine lentiviruses based on 104 new isolates: evidence for regular sheep-to-goat transmission and world-wide propagation through livestock trade. Virology. 2004;319(1):12-26.
- Shah C, Huder JB, Bo?ni J et al. Direct evidence for natural transmission of small- ruminant Lentiviruses of subtype A4 from goat to sheep and vice versa. J Virol. 2004;78(14):7518-7522.
- Staub, OC. Maedi-visna infection in sheep. History and present knowledge. Comp Immunol Microbiol Dis. 2004;27(1):1-5.