Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 9
1 November 2018
CASE II: H150787-83 (JPC 4085384-00).
Signalment: Dog (Canis lupus familiaris), intact female, 5-year-old, Chihuahua
History: The dog developed acute diarrhea and died suddenly. The dog was already dead at arrival to our teaching hospital and no further clinical investigations could be performed. The owner suspected intoxication and asked for a necropsy.
Gross Pathology: This dog was in good body condition but showed marked dehydration. The stomach contained two plastic fragments and the mucosa was brown to green. A thick viscous content was in the small intestine associated with a diffuse and moderately congested mucosa.
The cecum, colon and rectum were moderately thickened with a severely and diffusely congested mucosa. The content was scant, hemorrhagic and thick.
A concentric hypertrophy of the left ventricle, a moderate distal tracheal collapse, a discrete mesenteric lymph node hypertrophy and a mild meningeal congestion were also noted. All other organs and tissue were within normal gross limits.
Laboratory results: None given.
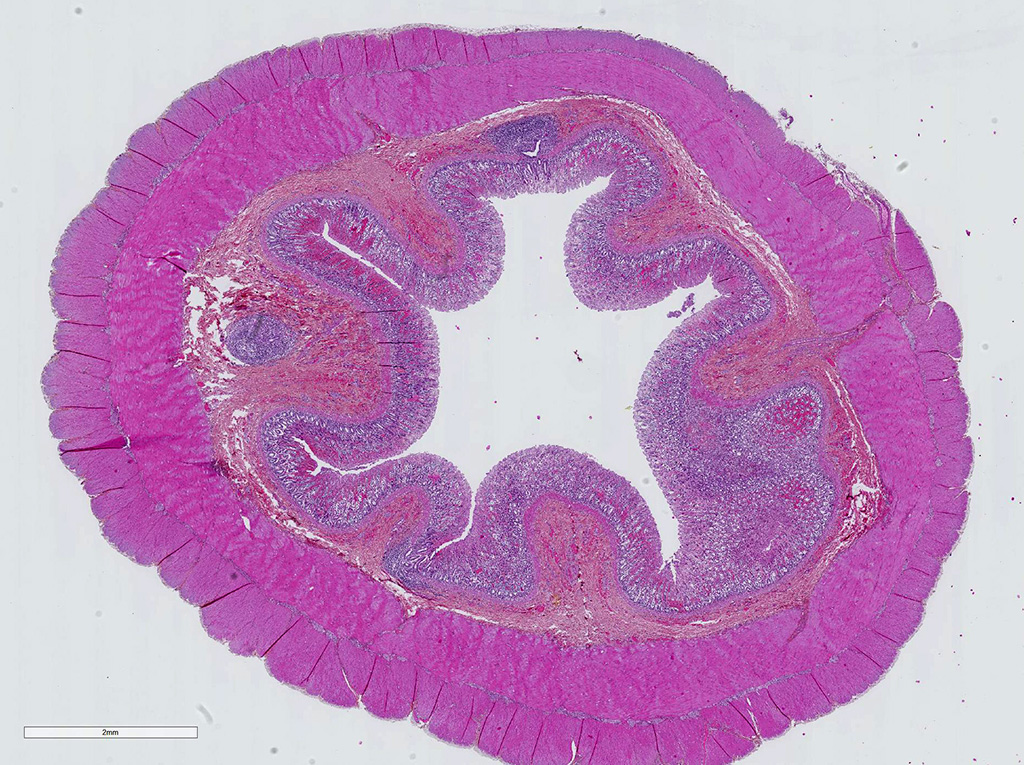
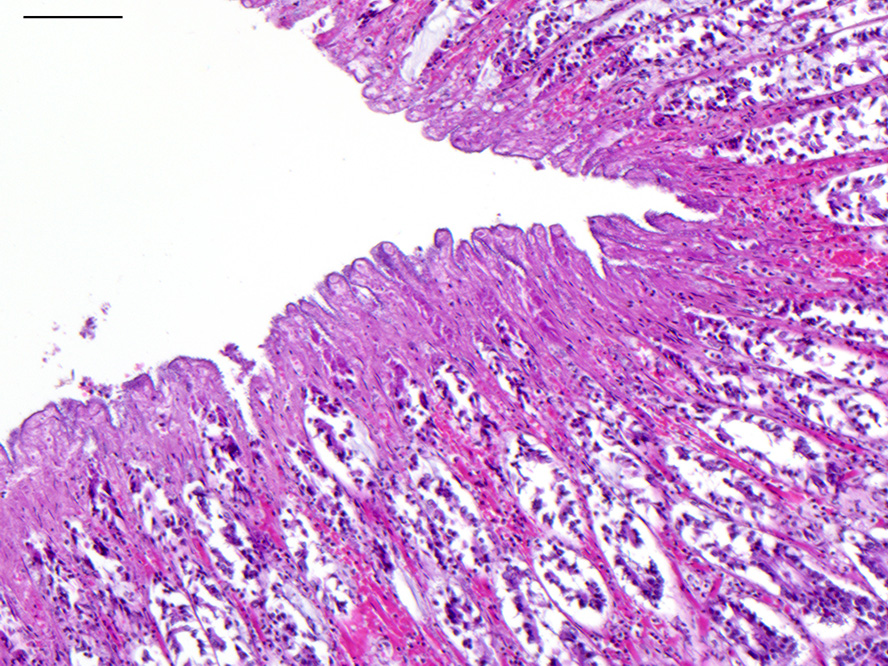
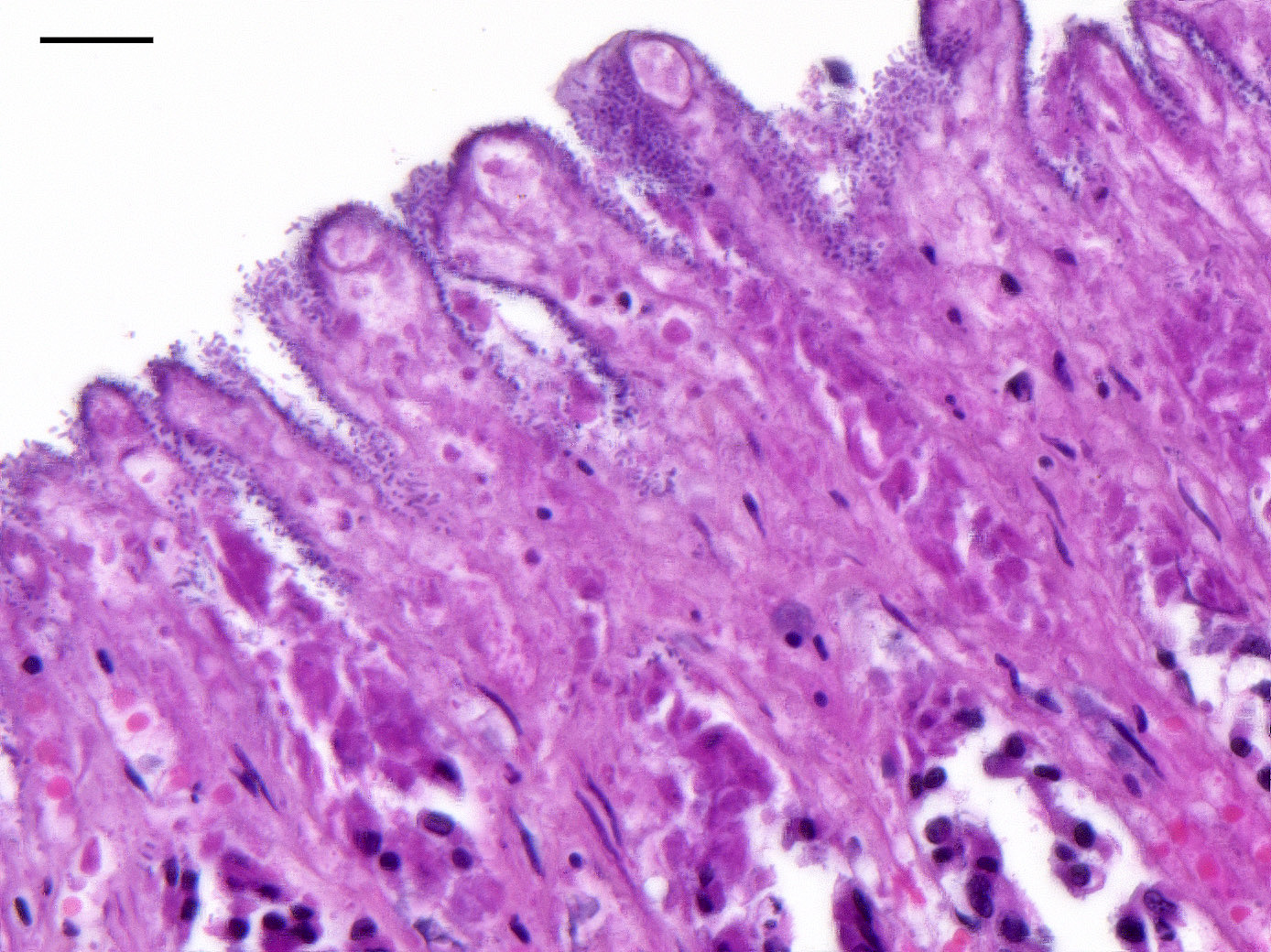
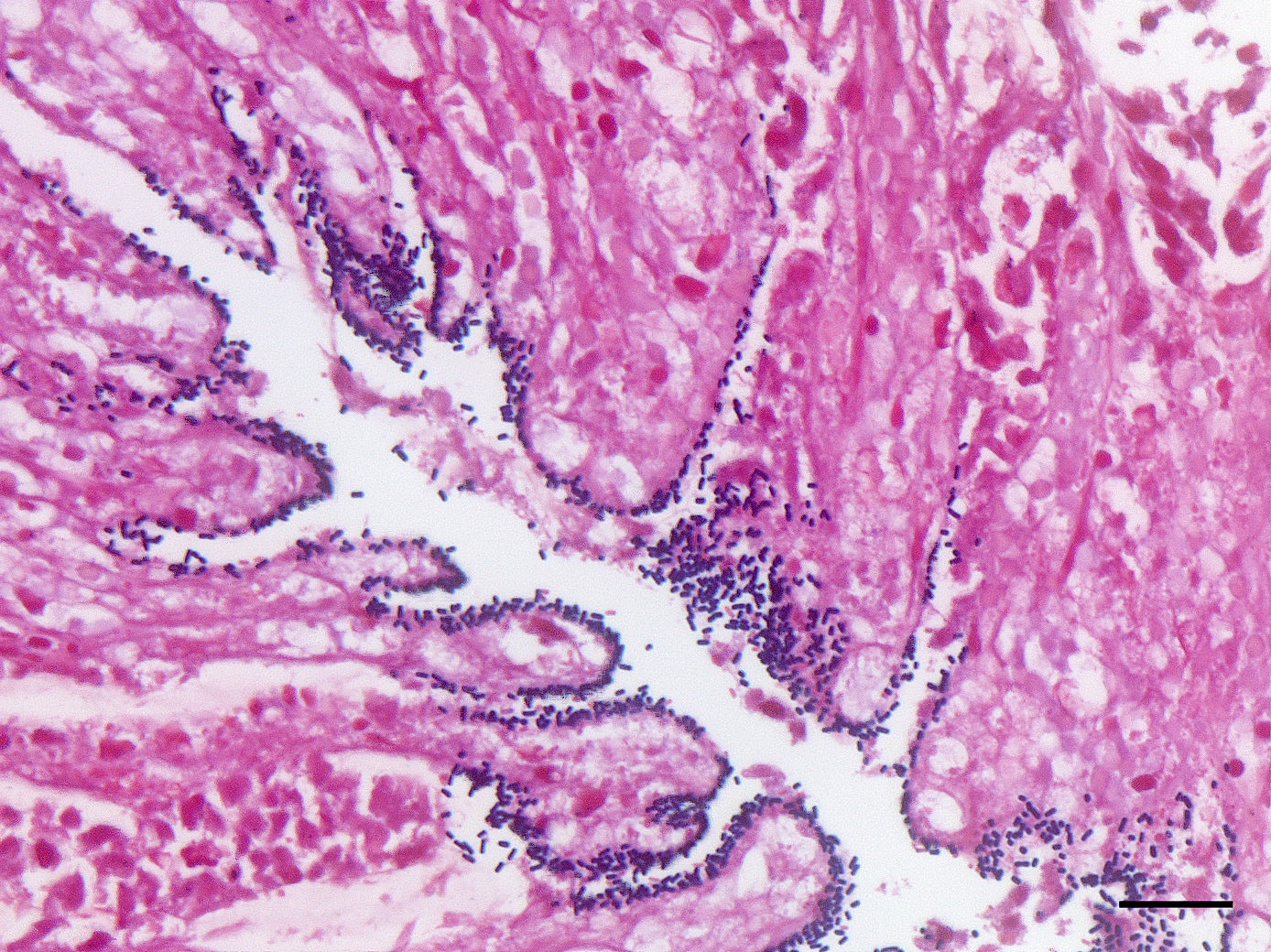
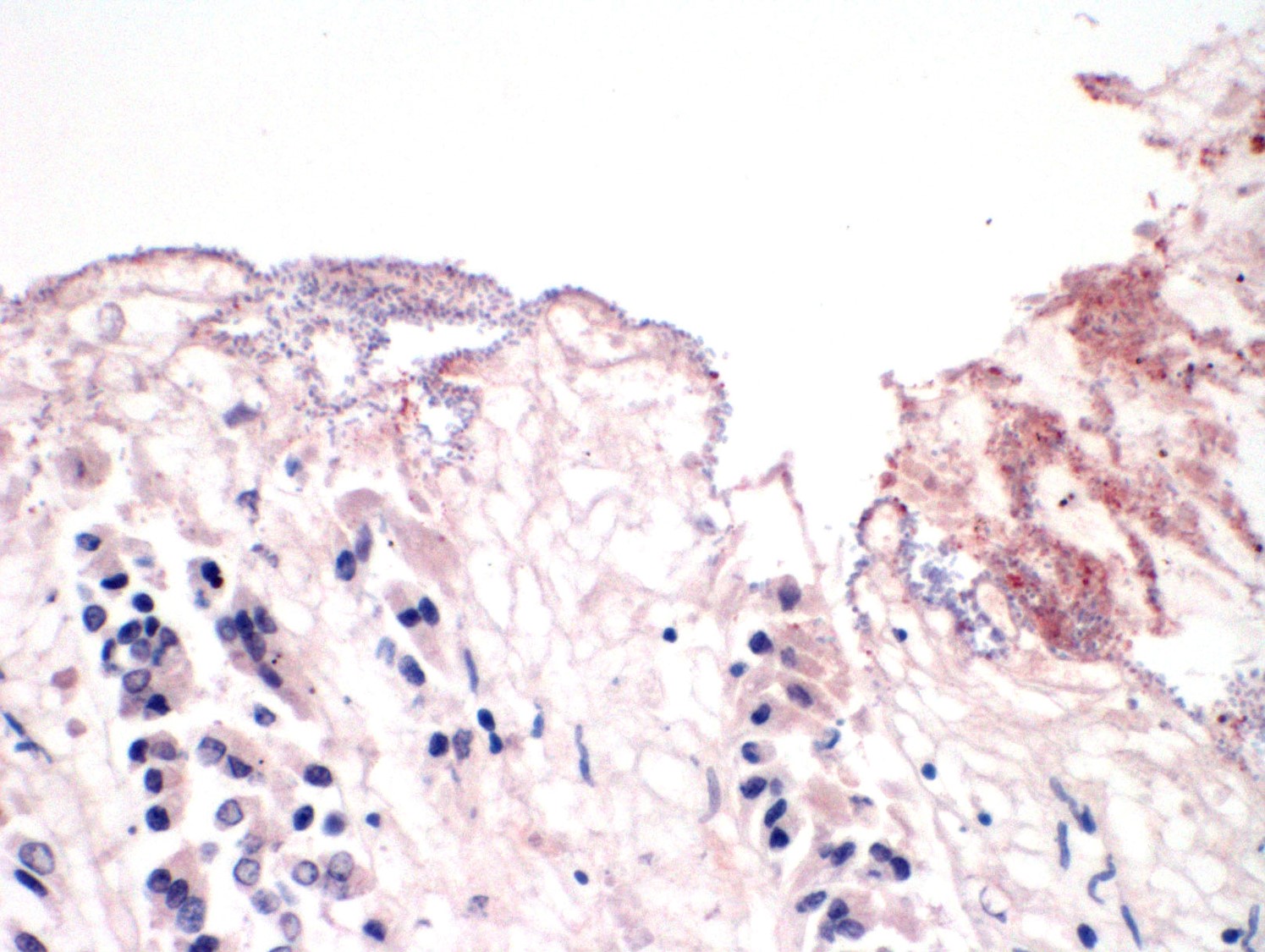
Microscopic Description: Colon: There is diffuse necrosis of the superficial mucosa, characterized by superficial mucosal hypereosinophilia with loss of cellular borders, pyknotic nuclei and loss of the mucosal epithelial lining. On the mucosal surface, admixed with necrotic debris, there are numerous 1-μm-long rod-shaped bacteria. The lamina propria and the submucosa present moderate to marked hyperemia and hemorrhage, and moderate infiltration by lymphocytes, plasma cells and degenerated neutrophils. There is multifocal thrombosis of small-sized blood vessels in the mucosa and submucosa.A Gram stain revealed numerous positive rods attached to the necrotic mucosal surface.
Contributor’s Morphologic Diagnoses:
Colon: Colitis, necrotizing and hemorrhagic, acute, diffuse, severe, with mucosal and submucosal vascular thrombosis and numerous gram-positive rod-shaped bacteria (consistent with Clostridium sp.) on to the mucosal surface
Name of the disease: Canine Hemorrhagic Gastroenteritis
Etiology: Clostridium perfringens type A (putative)
Contributor’s Comment: Canine Hemorrhagic Gastroenteritis (CHG) is a sporadic, peracute, hemorrhagic gastroenteritis of dogs. The etiology is not always identified, but Clostridium perfringens type A can been identified in some cases. A predilection for small breeds is described..6
Clostridium perfringens type A is responsible for mild and self-limiting diarrhea to fatal acute necrotizing and hemorrhagic enteritis in dogs and cats. It is also known to cause food poisoning in humans, necrotic enteritis in chickens, enterocolitis in horses and neonatal piglets, and enterotoxemia and hemorrhagic enteritis in lambs and calves.9
Clostridium perfringens is a gram-positive obligate anaerobic, spore-forming bacteria. It is part of the normal microbiota of human and animals, and a common enteric pathogen. It is separated into 5 types (A through E) based on the production of α, β, ε, and Ι toxins. Other important toxins, such as β2 and enterotoxin (CPE) may be produced by any of the five types, but the production of CPE is most commonly associated with type A strains.11
Clostridium perfringens type A major toxin is alpha toxin. Enterotoxin and β2 toxin may be produced by some strains. The bacteria require an anaerobic environment to sporulate and produce enterotoxin.11
CPE is a cytotoxic enterotoxin only elaborated during sporulation. It causes tissue damage and fluid secretion by binding to intestinal epithelial cells, forming pores in the plasma membrane that initiate cell death signaling pathway, and inducing structural damage to intercellular tight junctions resulting in increased epithelial permeability.4 However, the pathogenesis remains poorly understood because CPE can be demonstrated in the feces without sporulation and in the feces of healthy dogs.10
In 2015, three novel putative toxin genes encoding proteins related to the pore-forming Leukocidin/Hemolysin Superfamily were identified, and named netE, netF, and netG. Only netF was associated with cytotoxicity. There was a highly significant association between the presence of netF with type A strains isolated from cases of canine acute hemorrhagic gastroenteritis.4
Three predisposing factors for CHG are described: enteric infection by another pathogen (such as canine parvovirus), antibiotic administration, and disruption of the normal microbiota (for example by a sudden change to a high protein diet). Dogs present with hemorrhagic diarrhea. In the acute form, dogs are often found dead lying in a pool of blood excreta. Gross lesions consist of necrotizing and hemorrhagic enterocolitis, and sometimes gastritis. Lesions in the colon tend to be more severe, as in this case.
The histologic lesions consist of mural hemorrhagic necrosis of the gastrointestinal mucosa. Necrotic mucosal surface is lined by many gram-positive bacilli in association with fibrin and debris. The bacilli do not invade the lamina propria and can also be found within necrotic debris.10
Definitive diagnosis of CHG due to Cl. perfringens type A is not an easy task and cannot be based on macroscopic and microscopic lesions only. It requires combined testing for the CPE gene by PCR and for the Cl. perfringens enterotoxin by fecal enzyme-linked immunosorbent assay, and exclusion of other potential enteropathogens.11,13,16 In our case, such investigations could not be performed. However, the finding of an acute, fatal, necrotizing and hemorrhagic enterocolitis in a small-breed dog associated with numerous gram-positive bacilli is highly suggestive of CHG due to Cl. perfringens type A.
Contributing Institution:
Unité d’Histologie, d’Embryologie et d’Anatomie pathologique, Département des Sciences Biologiques et Pharmaceutiques, Ecole Nationale Vétérinaire d’Alfort, FRANCE : www.vet-alfort.fr
JPC Diagnosis: Colon: Colitis, necrohemorrhagic, diffuse, moderate, with thrombosis of submucosal vessels.
JPC Comment: The contributor provides an excellent review of this condition, which is well known to practitioners, but may be less well-known to veterinary pathologists. While the histologic lesion in this case is consistent with that seen in reported cases of acute canine hemorrhagic diarrhea syndrome (AHDS - a new and likely temporary name for this condition), the inability to either culture the pathogen or perform toxin identification is somewhat problematic in terms of a definitive identification of the etiologic agent. Mostly problematic is that the etiology remains unknown. C. difficile is identified in an equal or increased number of cases of hemorrhagic enteritis in dogs with similar predisposing factors to AHDS (previous antibiotic therapy, concurrent intestinal pathogens, and rapid dietary change.)3 However, C. difficile is found in similar prevalence in healthy dogs and its role in enteric canine disease remains undetermined.2
Since the submission of this case to the WSC, a review of lesions in endoscopic biopsies obtained from dogs with acute hemorrhagic diarrhea syndrome (a name chosen to reflect the lack of gastric lesions in affected animals) has been published.8 In endoscopic biopsies taken from 10 dogs with clinical AHDS, 9/10 demonstrate acute lesions of necrosis and neutrophilic infiltrate within the duodenum, 7/8 in the ileum, and 9/9 in the colon. Robust bacilli were identified in 5/6 duodenal samples, 5/8 ileal samples, and 7/9 colon samples.
C. perfringens type A was recently reported as a cause of necrotizing myositis in a German Shepherd Dog.12 A large mass of crepitant necrotic muscle developed on the right hindlimb over a period of 72 hours which necessitated radical surgical excision, drainage, and prolonged antibiotics; the animal survived. Multiplex PCR identified the agent in initial aspirates as Clostridium perfringens type A with a cpA toxin.12 C. perfringens type A is, beyond any doubt, responsible for human gas gangrene; alpha toxin is the main virulence factor of this type involved in gas gangrene.1,14
NetF is a novel toxin produced by some strains of C. perfringens type A. While preliminary evidence indicate that there seems to be a higher prevalence of NetF+ strains in dogs with AHDS than in healthy dogs2,4, final proof of the role of this toxin in this or other enteric conditions is lacking.
Upon review of the slide, the moderator discussed parameters which might be used in determining the presence of inflammation within the lamina propria versus a normal inflammatory population. While various criteria exist, but the separation of crypts and or lifting of crypts off of the muscularis mucosa by more than 3 layers of inflammatory cells suggests the presence of a true inflammatory infiltrate within the lamina propria.
Attendees discussed the possibility of parvoviral in this case based on morphologic finding, but the group consensus is that this differential diagnosis is not morphologically supported by the lack of profound crypt necrosis, necrosis of superficial mucosa and sparing of lymphoid tissue.
An immunohistochemical stain was performed for Clostridium perfringens type A at the San Bernardino lab of the California Animal Health and Food Safety system, and only a small percentage of the mucosal-adherent bacilli stained positively. The role of C. perfringens type A in enterocolitis and/or enterotoxemia of mammalian species is controversial and poorly supported by scientific evidence.15 The fact that C. perfringens type A has been identified in some cases (most cases actually) does not support a causal role in the disease, as this microorganism may be isolated from a large percentage of normal animals as well. One possible exception to the relationship between C. perfringens type A is perhaps yellow lamb disease.15 The contributor’s assertion that combined testing for the CPE gene by PCR and for the Cl. perfringens enterotoxin by fecal enzyme-linked immunosorbent assay would be helpful in this diagnosis is problematic as well in light of recent developments in the field as the production of C. perfringens enterotoxin is now restricted only to C. perfringens type F in humans, a common cause of food poisoning which has not be identified in animals.10
The consensus diagnosis of the attendees in this particular case is that, without any other submitted diagnostics, the etiology of this particular case remains in question.
References:
- Awad MM, Ellemor DM, Boyd RL, Emmins JJ, Rood Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene; Infect Immun. 2001 Dec;69(12):7904-10).
- Busch K, Suchodolski JS, Kühner KA, Minamoto Y, Steiner JM, Mueller RS, Hartmann K, UntererClostridium perfringens enterotoxin and Clostridium difficile toxin A/B do not play a role in acute haemorrhagic diarrhoea syndrome in dogs. Vet Rec 2015; Mar 7;176(10):253. doi: 10.1136/vr.102738.
- Diniz AN, Coura FM, Rupnik M, Adams V, Stent TI, Rood JI, de Oliveira Jr. CA, Lobator FCF, Silva ROS. The incidence of Clostridioides difficile and Clostridium perfringens netF-positive strains in diarrheic dogs. Anaerobe 2018; 49:58-62.
- Finley A, Gohari IM, Parreira VR, Abrahams M, Staempfli HR, Prescott JF. Prevalence of netF-positive Clostridium perfringens in foals in southwestern Ontario. Can J Vet Res. 2016; 80(3):242-4.
- Gohari IM, Parreira VR, Nowell VJ. A novel pore-forming toxin in Type A Clostridium perfringens is associated with both fatal canine hemorrhagic gastroenteritis and fatal foal necrotizing enterocolitis. PLOS ONE. 2015;10:e0122684.
- Iii JGS, Fisher DJ, Sayeed S, et al. The enteric toxins of Clostridium perfringens. in: Reviews of Physiology, Biochemistry and Pharmacology. Springer Berlin Heidelberg, 2004:183–204.
- Lawson PA, Citron DM, Tyrrell KL, Finegold SM. Reclassification of Clostridium difficile as Clostridioides difficile (Hall and O'Toole 1935) Prévot 1938. Anaerobe. 2016 Aug;40:95-9. doi: 10.1016/j.anaerobe.2016.06.008. Epub 2016 Jun 28.
- Leipid-Rudolph M, Busch K, Prescott JF, Gohari M, Leuteneggar CH, Hemranns W, Wolf G, Hartmann K, Verspohl J, Unterer S. Intestinal lesions in dogs with acute hemorrhagic diarrhea syndrome associated with InetF-positive Clostrium perfringens type A. J Vet Diagn Invest 2018; 30(4):495-503.
- Maxie G. Alimentary system. Jubb, Kennedy & Palmer’s Pathology of Domestic Animals. 6th ed. Saunders Ltd; 2015;2:183-185.
- Rood JI, Adams V, Lacey J, Lyras D, McClane BA, Melville SB, Moore RJ, Popoff MR, Sarker MR, Songer JG, Uzal FA, Van Immerseel F. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe; 2018 pii: S1075-9964(18)30068-4. doi: 10.1016/j.anaerobe.2018.04.011.
- Schlegel BJ, Van Dreumel T, Slavi? D. et al. Clostridium perfringens type A fatal acute hemorrhagic gastroenteritis in a dog. Vet. J. 2012;53:555-557.
- Sedigh HS, Rajabloun M, Razmyar J, Mehrjerdi HK. An unusual necrotic myositis by Clostridium perfringens in a German Shepherd dog: a clinical report, bacteriological and molecular identification.
- Silva ROS, Lobato FC. Clostridium perfringens: A review of enteric diseases in dogs, cats and wild animals. Anaerobe. 2015;33:14–17.
- Uzal FA, McClane BA, Cheung JK, Theoret J, Garcia JP, Moore RJ, Rood Animal models to study the pathogenesis of human and animal Clostridium perfringens infections.Vet Microbiol. 2015 Aug 31;179(1-2):23-33. doi: 10.1016/j.vetmic.2015.02.013.
- Uzal FA, Plattner BL, Hostetter JM. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier;2016:216-217.
- Washabau RJ, Day MJ. Disease of the gastrointestinal tract. In: Canine and Feline Gastroenterology, Saunders; 2012.