WSC 2023-2024, Conference 16, Case 2
Signalment:
2-month-old male rose-ringed parakeet (Psittacula krameri)
History:
Sudden death without any clinical signs of disease.
Gross Pathology:
Necropsy revealed sparse feathers in the abdomen and back areas, multifocal subcutaneous hemorrhage and hemorrhage in pectoral muscles and muscles of extremities and extensive hepatic necrosis and hemorrhage.
Laboratory Results:
PCR testing for avian polyomavirus was positive; PCR testing for psittacine beak and feather disease, avian bornavirus, herpesvirus, and Chlamydia was negative.
Microscopic Description:
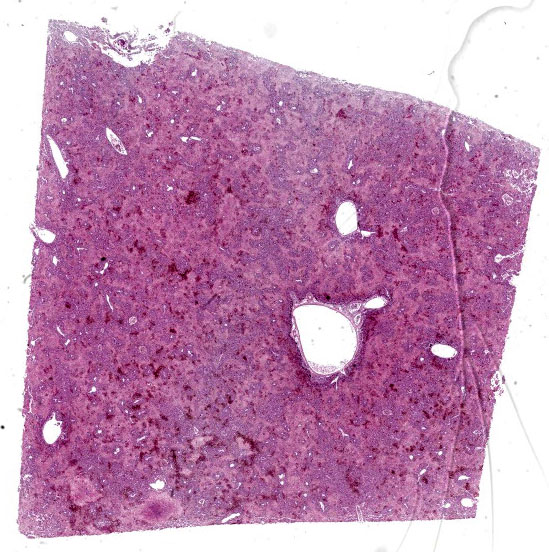
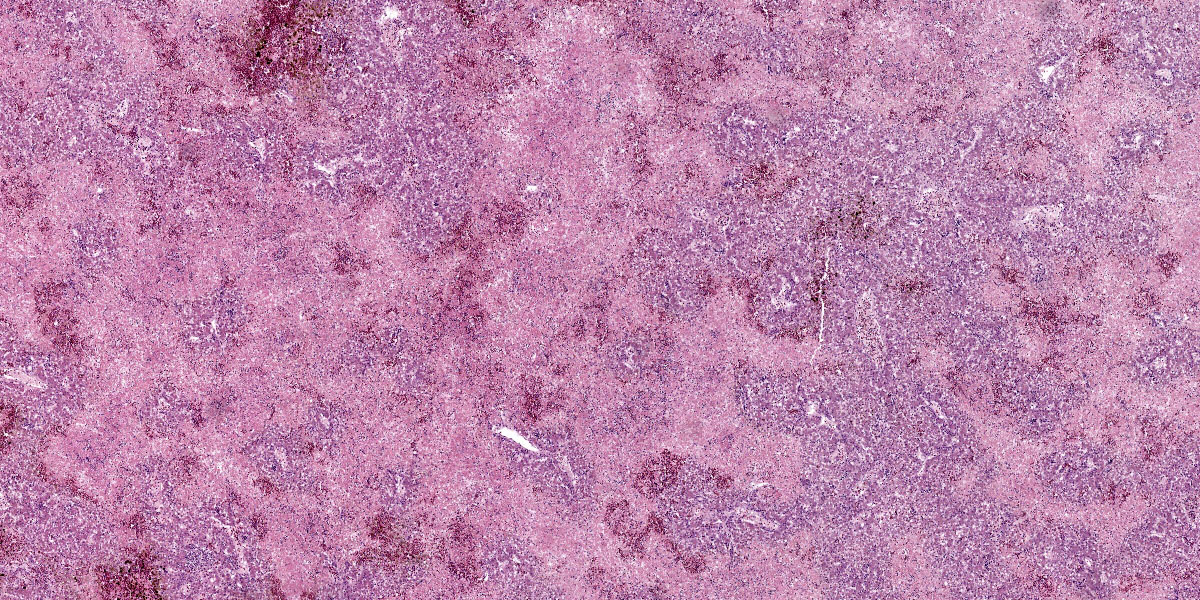
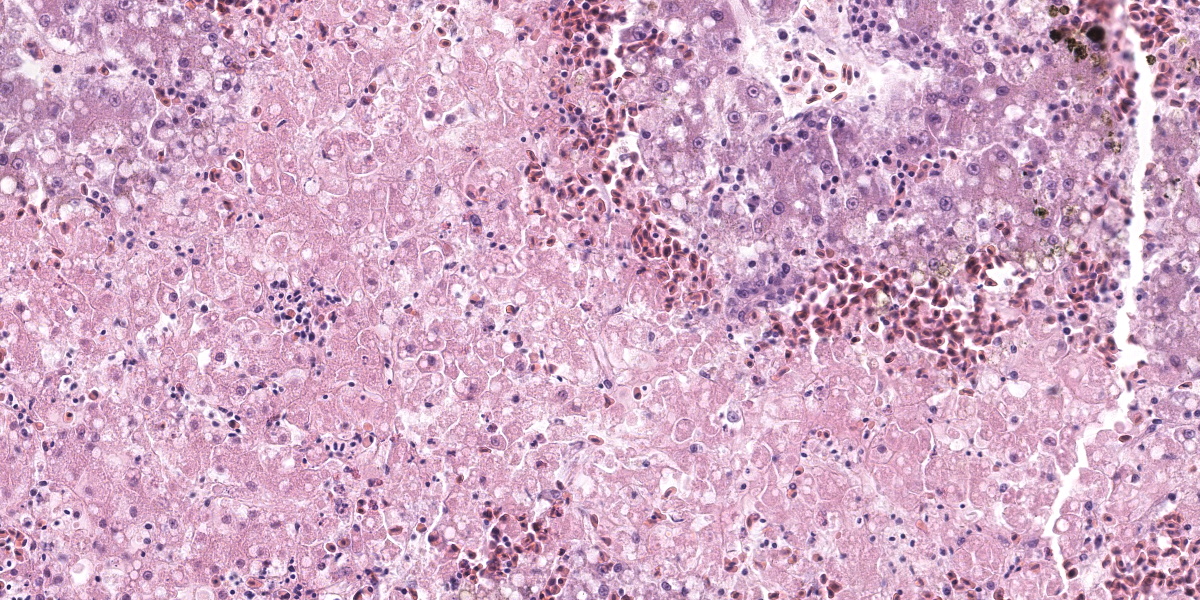
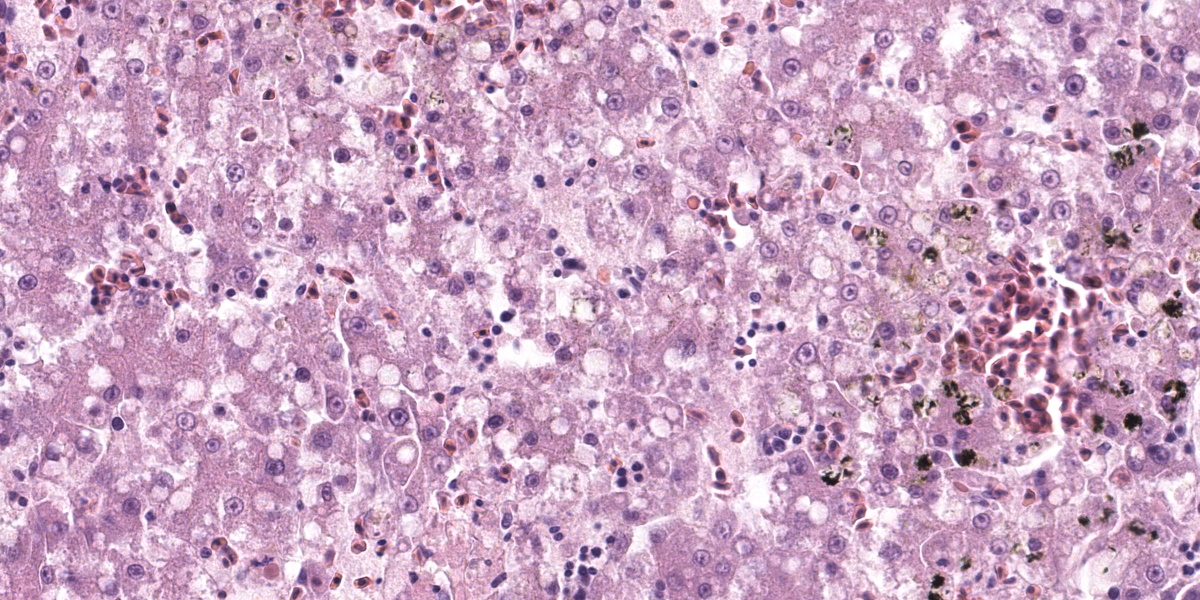
Liver: Affecting up to 70% (this percentage somewhat varies between the slides) of the liver parenchyma there is disruption and loss of the hepatic architecture evident by either of the following: loss of differential staining and paleness of hepatocytes with karyolysis (lytic necrosis), eosinophilic cellular and karyorrhectic debris with hypereosinophilic cytoplasm and pyknosis (coagulative necrosis), and moderate numbers of extravasated erythrocytes (hemorrhage). Such changes affect mostly centrilobular and midzonal portions of the lobules and frequently coalesce (centrilobular and midzonal necrosis). Adjacent hepatocytes are frequently swollen with pale vacuolated cytoplasm (hepatocellular degeneration). Rarely such hepatocytes have mildly enlarged nuclei that contain large amphophilic to lightly eosinophilic, irregularly round inculsion bodies that completely efface the nuclear chromatin and peripheralize the nuceloli. Multifocally within the portal areas there is an increase in the number of bile duct profiles (ductular reaction). Scattered throughout the sinusoids are uncommon aggregates of hematopoietic cells with erythroid and fewer myeloid precursors (extramedullary hematopoiesis).
Contributor’s Morphologic Diagnosis:
Liver: Hepatocellular necrosis and degeneration, acute, multifocal to coalescing, submassive, severe, with rare large intranuclear amphophilic viral inclusion bodies.
Contributor’s Comment:
Avian polyoma virus (APV) is one of the most significant viral pathogens of caged birds. It results in substantial economic losses for aviculturalists and pet store owners each year. APV is included in the genus Polyomavirus, family Papovaviridae. Its ability to infect and cause disease in birds is dependent on the age of the bird, the species, the immune status of the bird, and other poorly understood factors.6
APV was first characterized as a pathogen in young budgerigars in 1981 and was designated budgerigar fledgling disease virus.1 Now it is called avian polyomavirus because it affects many different species of psittacine birds. To date, four polyomaviruses in birds are known, namely avian polyomavirus, goose hemorrhagic polyomavirus (GHPV), finch polyomavirus (FPyV) and crow polyomavirus (CPyV).
In fledgling and young budgerigars, APV causes acute death, abdominal distention and feather abnormalities known as “French molt.” It also causes a loss of down feathers on the back and abdomen, filoplumes on the head and neck, and subcutaneous hemorrhage of nesting budgerigars. In other avian orders, including psittacine species, APV causes clinical signs similar to those observed in budgerigars, but the severity of the disease seems to be dependent on the species infected.4 Other species of psittacine birds can be very ill at weaning with nonspecific weight loss, anorexia, partial paralysis of the gut, polyuria, and watery droppings. They have a tendency to haemorrhage easily, and may have CNS signs. Not all the birds get the disease and not all of those affected die; some (especially the older birds) recover to become asymptomatic carriers.2
Typical pathologic changes in psittacine birds include hydropericardium, enlarged heart, swollen liver, congested kidneys, and hemorrhage within the body cavities. Histopathology typically reveals large and slightly basophilic intranuclear inclusion bodies in various tissues, especially in the spleen, liver, and kidneys.4
Recent references about APV are mainly related to monitoring of free-ranging and captive birds for polyomavirus and other avian viruses. Kessler et al., 2020, demonstrated that the prevalence of polyomavirus in free-ranging Psittacula populations in Europe is low despite the general susceptibility of these species to this virus.5 Rahaus and Wolff, 2005, conducted a survey to detect subclinical poly-omavirus infections in captive psittacine birds in Germany and they did not detect APV in any samples.8 However, surveys in Australia and Poland detected significant rates of infection in captive parrot populations with APV.3,7 All of this emphasizes the need to adhere to strict biosecurity measures and regular testing for common psittacine pathogens especially in rehabilitation centers and breeding units.
Contributing Institution:
Department of Veterinary Pathology
Faculty of Veterinary Medicine
University of Zagreb
Heinzelova 55
10000 Zagreb, Croatia
http://www.vef.unizg.hr/
JPC Diagnosis:
Liver: Necrosis, coagulative, multifocal to coalescing, severe, with mild to moderate hepatocellular lipidosis.
JPC Comment:
The contributor provdes an excellent overview of avian polyomavirus, an important avian disease that exhibits a wide variety of case presentations ranging from subclinical disease tosudden death, depending on species susceptibility. Conference participants found this case particularly rewarding as it provide ample fodder for discussion of foundational issues in liver pathology, such as lesion distribution and the association between lesion distribution and possible etiologies.
In this liver, the distribution of the main lesion - striking, large areas of necrosis – was the main point of discussion. Dr. Pesavento began with a subgross evaluation of the distribution, which many participants felt was zonal, while others felt that the distribution, while geographic and incorporating wide swaths of tissue, was essentially random. Dr. Pesavento noted that every lobule of the liver appears to be affected, which argues against a random distribution, but agreed that the distribution of hepatic lesions in birds can generally be difficult to determine, as this case demonstrates.
The majority of participants agreed that the lesion appeared concentrated in the centrilobular and midzonal areas. Due to this zonality and the diffuse necrosis throughout all liver lobules in section, participants felt that insults expected to have a zonal distribution, such as hypoxia and toxin exposure, should be included in the differential diagnosis list. Other differentials suggested by participants included viral causes, such as psittacid herpesvirus 1, and avian polyomavirus.
Dr. Pesavento discussed the reticulin-staining in this liver, contrasting it with the rabbit liver in the previous case. In this bird, the reticulin framework appeared intact, indicating a more acute process. The intact reticulin network also suggests that the observed necrosis is affecting hepatocytes, not endothelial cells. This suggestion is bolstered by the reduced distance between the intact peri-sinusoidal reticulin networks, which is an indicator of hepatocellular loss.
Returning to the H&E slide and the proposed polyomaviral etiology, participants noted the lack of convincing polyomaviral inclusions, which are typically large, intranuclear, basophilic to amphophilic features which can substantially obscure the nucleus. Polyomaviral infections typically generate many of these dramatic inclusions and participants found their absence surprising. Participants were also perplexed by the zonal, non-random lesion distribution, which is unusual for a viral infection. The moderator noted that subclinical polyomaviral infections are relatively common, raising the possibility that this animal’s lesions were not caused by polyomavirus, despite being PCR-positive for the agent.
Conference participants discussed what additional diagnostics could be used to investigate possible etiologic agents. Participants felt that Giemsa or Gram staining would be helpful to rule out septicemia, and that in situ hybridization (ISH) would be helpful to confirm the presence of polyomavirus in the liver and to determine if the virus is co-localized with areas of necrosis. Finally, participants noted that avian polyomavirus inclusions are most reliably found in the spleen, adding an H&E section of splenic tissue to the diagnostic wish list.
Despite being open to alterate etiologies for the observed lesions, conference participants largely agreed with the contributor’s morphologic diagnosis. No lesion distribution was included in the JPC diagnosis as participants remained equivocal as to whether the lesions were random or zonal, and despite intense searching, no intranuclear inclusions were observed in the section examined in conference.
References:
- Davis RB, Bozeman LH, Gaudry D, Fletcher OJ, Lukert PD, Dykstra MJ. A viral disease of fledgling Budgerigars. Avian Dis. 1981;25(1):179-183.
- Harcoutt-Brown NH. Psittacine birds. In: Tully TN, Dorrestein GM, Jones AK, eds. Handbook of Avian Medicine. 2nd ed. Saunders Elsevier; 2000:138.
- Hulbert CL, Chamings A, Hewson KA, Steer PA, Gosbell M, Noormohammadi AH. Survey of captive parrot populations around Port Phillip Bay, Victoria, Australia, for psittacine beak and feather disease virus, avian polyomavirus and psittacine adenovirus. Aust Vet J. 2015;93 (8):287-292.
- Katoh H, Ogawa H, Ohya K, Fukushi H. A Review of DNA Viral Infections in Psittacine Birds. J Vet Med Sci. 2010;72(9):1099-1106.
- Kessler S, Heenemann K, Krause T, et al. Monitoring of free-ranging and captive Psittacula populations in Western Europe for avian bornaviruses, circoviruses and polyomaviruses. Avian Pathol. 2020;49 (2):119-130.
- Phalen DN. Avian Polyomavirus: My Thoughts. Alfa Watchbirb. 1998;25(5):28-39.
- Piasecki T, Wieliczko A. Detection of beak and feather disease virus and avian polyomavirus DNA in Psittacine birds in Poland. Bull Vet Inst Pulawy. 2010;54: 141-146.
- Rahaus M, Wolff MH. A survey to detect subclinical polyomavirus infections of captive psittacine birds in Germany. Vet Microbiol. 2005;105:73-76.