Signalment:
Gross Description:
Cytology:
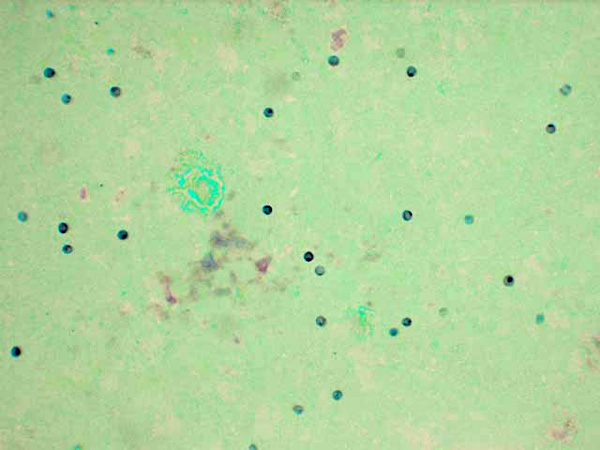
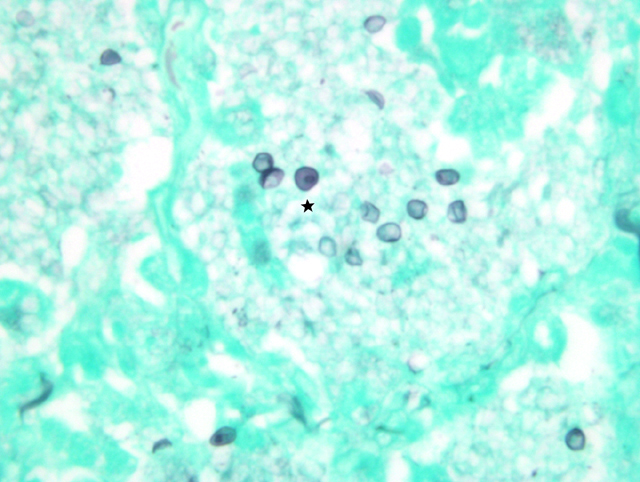
Lung imprints were prepared using a cut margin of lung stained with a modified Wright-Giemsa stain. Cytologic examination revealed red blood cells, alveolar macrophages, type II pneumocytes, neutrophils, and numerous round to oval extracellular organisms measuring approximately 4 _m in diameter. Nuclei were evident within the organisms and were consistent with the cystic and trophozoite forms of Pneumocystis carinii.Â
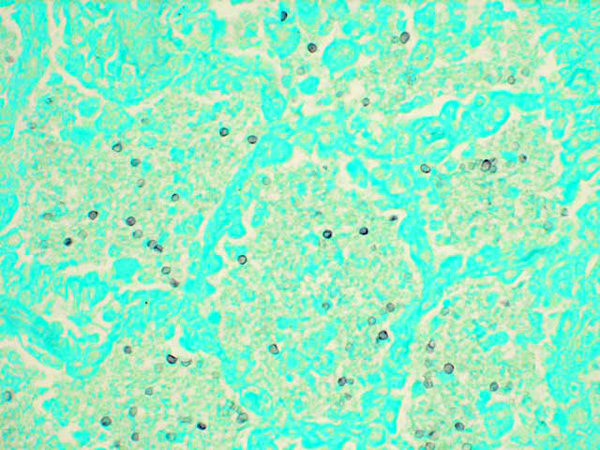
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
There are 5 host-specific species of Pneumocystis: Pneumocystis carinii, Pneumocystis jiroveccii, Pneumocystis murina, Pneumocystis oryctolagi, and Pneumocystis wakefieldiae. Pneumocystis carinii species infects SIV positive macaques and the Pneumocystis jiroveccii species infects HIV positive humans.1, 7, 12 Wild born and laboratory bred macaques have similar susceptibilities to Pneumocystis carinii, however, wild born macaques exhibit higher levels of antibody titers to the fungus.8 The first report of infection in nonhuman primates included two aged owl monkeys and two young chimpanzees with myeloproliferative neoplasia.3
Lungs affected with severe Pneumocystis pneumonia typically exhibit alveolar type-II cell hypertrophy and intra-alveolar foamy eosinophilic material and variable neutrophilic lung inflammation that may result in diffuse alveolar damage, impaired gas exchange, and respiratory failure.6, 14 The Pneumocystis fungi attach to type-I alveolar epithelial cells and, although the organisms do not alter the metabolic, barrier, or structural functions of the alveolar cells, they are able to proliferate due to impaired cell-mediated immunity. Infection also leads to changes in pulmonary surfactant production initiated by the inhibition of phosphatidylcholine from type II alveolar cells.6 Pneumocystis organisms exist as cystic and haploid trophozoite forms throughout their life-cycle, but during infection, the trophozoite form is more abundant than the cystic form.14
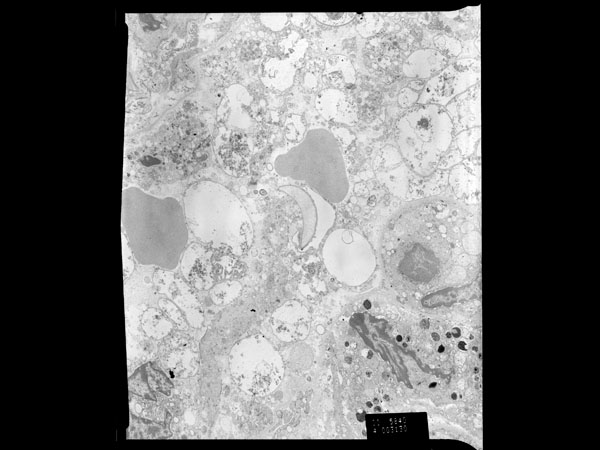
In the transmission electron micrographs of a thin section of Pneumocystis infected lung from this macaque, cyst and trophozoite forms are evident with trophozoites predominating. Free ribosomes and glycogen are present in both forms. Cysts are typically spherical in shape, contain up to eight intracystic bodies from which trophozoites originate, and have a mean diameter of 5-8 μm. The mean diameter of an intracystic body is 1.2 μm.5 Each intracystic body contains components characteristic of eukaryotic cells including a centrally located nucleus, endoplasmic reticulum, and other organelles. The cyst wall is two layers thick, measuring approximately 50 nm.5 Trophozoites are generally grouped into an interdigitating cluster, are individually pleomorphic, and are closely associated with pneumocytes. There are very few organelles, including a nucleus with a centrally to peripherally placed nucleolus. Free ribosomes predominate within the infective form. A 20-30 nm thick, non-homogenous coat surrounds the entire trophozoite, including the interdigitations, and is a characteristic feature of the trophozoite form. The coat may help anchor the trophozoite to other trophozoites or alveolar epithelium or it may be involved in nutrient uptake.5
Trophozoites adhere to the alveolar epithelium to establish infection and to initiate a mitogen activated protein kinase signaling cascade for mating and proliferation of the fungus.14 CD4+ T lymphocytes are integral in the defense against Pneumocystis.6 By recruiting and activating macrophages and monocytes to attack the invading organism, CD4+ cells behave as memory cells to initiate the hosts immune response.14 Continuously declining CD4+ levels in this animal to <3% of cells/μL of blood allowed the Pneumocystis organisms to extensively proliferate and exert their adverse effects. SIV positive monkeys infected with Pneumocystis are unable to recruit CD4+ cells to the lung and develop an inflammatory response characterized by an increased neutrophil and CD8+ cell infiltration.4
Not all of the imposed alveolar damage is due to the adherence of Pneumocystis organism to the type-I alveolar epithelial cells. TNF-_, an inflammatory cytokine that regulates the immune response by recruiting neutrophils, lymphocytes, and monocytes to an area of infection, is also a contributing factor in damage to the alveoli. While TNF-_ helps mount an immune response, it also releases oxidants, cationic proteins, and proteases responsible for subsequent damage to pulmonary tissue.14 Trimethoprim-sulfamethoxazole combined with corticosteroid therapy to suppress lung inflammation is the preferred and most effective course of treatment for Pneumocystis and is usually begun when the CD4+ cell count is less than 200 per cubic millimeter, a state of a heightened risk of infection.14 Adverse effects of the drug are common, however, and use of this prophylaxis has rapidly increased the multidrug resistance of infection bacterial pathogens found in human immunodeficiency virus-infected animals.10, 14
JPC Diagnosis:
1. Lung: Pneumonia, interstitial, histiocytic and neutrophilic, chronic, diffuse, moderate, with type II pneumocyte hyperplasia, multinucleate giant cells, and myriad intraalveolar fungi, etiology consistent with Pneumocystis carinii, Rhesus macaque (Macaca mulatta), primate.
2. Cytological specimen, impression smear, lung: Numerous epithelial cells, macrophages with vacuolated cytoplasm, few neutrophils, and myriad 3-5um round cysts containing punctuate organisms (trophic bodies) on a blue, granular, proteinaceous background.Â
Conference Comment:
References:
2. Caswell JL, Williams KJ: Respiratory system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 2, p. 593. Elsevier Limited, St. Louis, MO, 2007
3. Chandler FW, McClure HM, Campbell WG, Watts JC: Pulmonary pneumocystosis in nonhuman primates. Arch Pathol Lab Med 100:163-167, 1976
4. Croix DA, Board K, Capuano S, Murphey-Corb M, Haidaris CG, Flynn JL, Reinhart T, Norris KA: Alterations in T lymphocyte profiles of bronchoalveolar lavage fluid from SIV- and Pneumocystis carinii-coinfected Rhesus macaques. AIDS Res Hum Retroviruses 18:391-401, 2002
5. de Souza W, Benchimol M: Basic biology of Pneumocystis carinii-a mini review. Mem Inst Oswaldo Cruz 100:903-908, 2005
6. Dei-Cas E: Pneumocystis infections: the iceberg? Med Mycol 38:23-32, 2000
7. DeManche C, Berthelemy M, Petit T, Polack B, Wakefield AE, Dei-Cas E, Guillot J: Phylogeny of Pneumocystis carinii from 18 primate species confirms host specificity and suggests coevolution. J Clin Microbiol 39:2126-2133, 2001
8. Fujita M, Furuta T, Kojima S, Kurata T, Yoshikawa Y: Survey for Pneumocystis carinii infection of wild-born and laboratory-bred monkeys by indirect immunofluorescences and cyst-staining methods. Jpn J Med Sci Biol 49:113-120, 1996
9. Furuta T, Fujita M, Mukai R, Sakakibara I, Sata T, Miki K, Hayami M, Kojima S, Yoshikawa Y: Severe pulmonary pneumocystosis in simian acquired immunodeficiency syndrome induced by simian immunodeficiency virus: its characterization by the polymerase-chain-reaction method and failure of experimental transmission to immunodeficiency animals. Parasitol Res 79:624-628, 1993
10. Huovinen P: Resistance to trimethoprim-sulfamethoxazole. Clin Infect Dis 32:1608-1614, 2001
11. L³pez A: Respiratory system. In: Pathologic Basis of Veterinary Disease, eds. McGavin MD, Zachary JF, 4th ed., pp. 471, 536, 546. Elsevier, St. Louis, MO, 2007
12. Medrano FJ, Montes-Cano M, Conde M, de la Horra C, Respaldiza N, Gasch A, Perez-Lozano MJ, Varela JM, Calderon EJ: Pneumocystis jirovecii in general population. Emerg Infect Dis 11:245-250, 2005
13. Stahl J, Sage MR: Radiological-pathological correlation: Alveolar pattern. Australasian Radiology 45:74-97, 2001
14. Thomas CF, Limper AH: Pneumocystis pneumonia. N Engl J Med 350:2487-98, 2004
15. Vogel P, Miller CJ, Lowenstine LL, Lackner AA: Evidence of horizontal transmission of Pneumocystis carinii pneumonia in simian immunodeficiency virus-infected Rhesus macaques. J Infect Dis 168:836-843, 1993