Signalment:
Gross Description:
Morphologic Diagnosis:
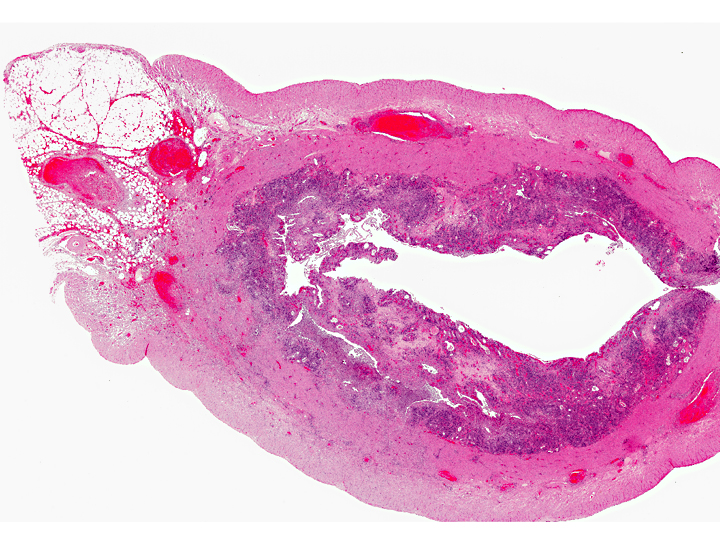
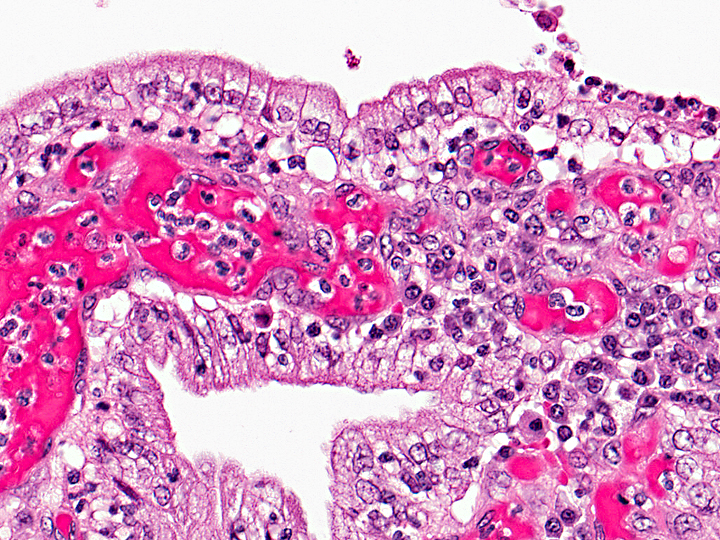
Uterus: Cystic hyperplasia, endometrial, moderate
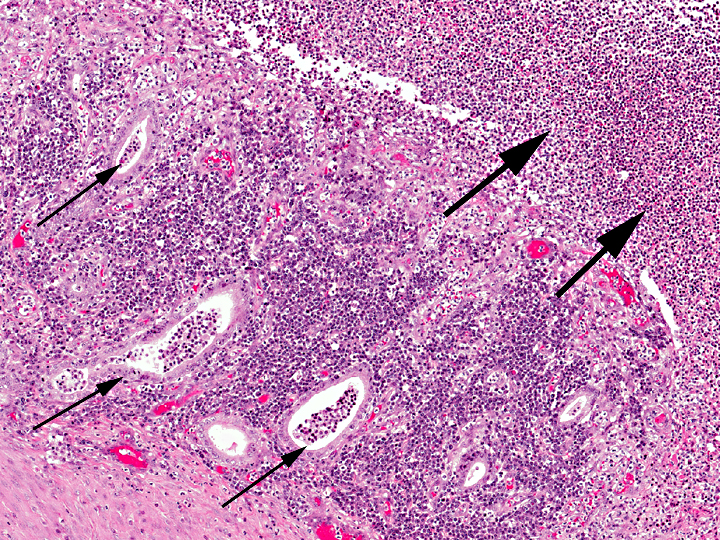
Uterus: Necrosuppurative and lymphoplasmacytic endometritis, marked, with hemorrhage
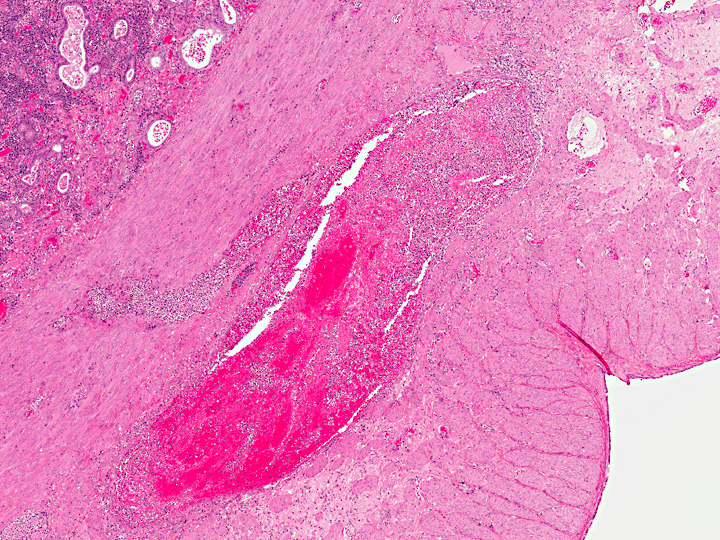
Uterus, myometrium: Vasculitis, necrosuppurative, with thrombus formation, marked
Condition:
Contributor Comment:
CEH most commonly occurs in intact bitches (most frequency in those animals that have not undergone pregnancies) and its pathogenesis is related to estrogen stimulation of the uterus followed by prolonged exposure to progesterone during diestrus. Progesterone acts to stimulate endometrial glandular secretion and to suppress contractions of the uterus, thus creating a uterine environment predisposed to bacterial growth(11). Although CEH has long been thought to be a necessary predisposing factor for pyometra and certainly they are often observed together as in this case, each can occur independently of the other. New evidence suggests that pyometra should be considered a separate entity from CEH since CEH relies entirely on a hormone pathogenesis while pyometra is ultimately triggered by bacterial infection(4,11). The most common bacterium isolated in canine pyometra is Escherichia coli, which is isolated in 59-96% of cases(4). Endotoxin derived from the etiologic agent (which is most often a gram-negative bacteria) often results in sepsis, manifested by numerous cardiovascular, gastrointestinal, and overwhelming systemic inflammatory findings. In the absence of surgical intervention, death is common(4).
The typical clinical presentation of pyometra is inappetance, depression, polydipsia, lethargy, abdominal distension due to an enlarged uterus, +/- vaginal discharge. Marked leukocytosis is the most common hematologic finding, although anemia, hyperglobulinemia, hypoalbuminemia, and elevation of markers of intrahepatic cholestasis are also often observed(4,11). The incidence of pyometra in a colony of intact female beagles >4 years of age was 15%, and incidence increased with age(5). Despite its bacterial component, antibiotic treatment is rarely effective. Ovariohysterectomy is the most reliable and successful treatment for canine pyometra(5).
JPC Diagnosis:
Conference Comment:
The contributor mentioned the clinicopathologic finding of intrahepatic cholestasis, which is increased alkaline phosphatase, bilirubin, and cholesterol with an ALT within normal limits, which differentiates the findings from hepatocellular necrosis(3).
Death from pyometra is often due to systemic inflammatory response syndrome (SIRS), resulting in the release of inflammatory mediators and the activation of neutrophils and platelets, resulting in multiple organs dysfunction syndrome and shock. Clinical criteria for diagnosing SIRS in dogs are at least two of the following: heart rate greater than 160 beats per minute, body temperature greater than 103.5°F or less than 100°F, respiratory rate of greater than 20 breaths per minute, pCO2 of less than 32, or a leukocyte count of greater than 12,000 or less than 4,000 or greater than 10% bands(9).
SIRS caused by the lipopolysaccharide (LPS) endotoxin, as in this case, causes tachycardia and tachypnea in lower doses, and hemorrhagic diarrhea, vomiting, myocardial failure, and death in high doses. LPS binds lipopolysaccaride binding protein (LBP) in serum which in turn binds cell surface pattern-recognition receptor CD14. This activates clotting factor XII (Hageman factor), and induces toll-like receptor (TLR) 4. Also, the endothelium is activated, and the anticoagulant tissue factor production inhibitor (TFPI) and thrombomodulin are inhibited. Monocytes and macrophages are activated, releasing tumor necrosis factor alpha (TNF), IL-1, IL-6, IL-8, and other chemokines, which induces further inflammation and incites the release of acute phase proteins from the liver, such as LBP, mannose-binding protein, fibrinogen, C-reactive protein, serum amyloid A (SAA), hepatoglobin, hepcidin, C3 and C4, alpha-1 antitrypsin and alpha-1 acid glycoprotein. Many of the acute phase proteins bind bacterial components, such as the cell wall, and act as opsonins and fix complement. Some proteins that are reduced, also known as negative acute phase proteins, are transferrin, albumin, pre-albumin, antithrombin, and alpha-2 macroglobulin (in cattle). Also, the complement cascade is directly activated, generating C3a and C5a(6,7,8).Â
Animals that survive SIRS are predisposed to compensatory anti-inflammatory response syndrome (CARS), leading to decreased macrophage activity, T cell anergy, and apoptosis of lymphocytes(2).
Conference participants also discussed some causes of endometrial hyperplasia, such as prolonged hyperestrogenism due to subterranean and red clover in ewes, which causes reduced fertility, dystocia, uterine prolapse due to hypotonicity, endometrial gland development in the cervix, and mammary gland engorgement. Sows develop endometrial cysts due to zearalenone mycotoxicosis, and cows develop cystic follicles and granulosa cell tumors from prolonged plant estrogen ingestion(10).
References:
2. Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemostat. 2009; 101(1):36-47.
3. Bain PJ. Liver. In: Latimer KS, ed. Duncan & Prasses Veterinary Laboratory Medicine: Clinical Pathology. Ames, IA:Wiley-Blackwell; 2011:212, 218.
4. Fransson BA, Ragle CA: Canine Pyometra: An Update on Pathogenesis and Treatment. Compendium 25:602-612, 2003
5. Fukuda S: Incidence of Pyometra in Colony-raised Beagle Dogs. Exp. Anim. 50: 325-329, 2001
6. Joles JA, Malnix JA. Polydipsia and polyuria. In: Kirk RW ed. Current Veterinary Therapy. Vol. 6 Philadelphia, PA:Saunders; 1977.
7. Kumar V, Abbas AK, Fausto N, Aster JC. Acute and chronic inflammation. In: Kumar V, Abbas AK, Fausto N, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA:Saunders Elsevier; 2010:45-75.
8. Mitchell RN. Hemodynamic disorders, thromboembolic disease, and shock. In: Kumar V, Abbas AK, Fausto N, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA:Saunders Elsevier; 2010:111-32.
9. Purvis D, Kirby R. Systemic inflammatory response syndrome: septic shock. Vet Clin North Am. 1994; 24(6):12251247.
10. Schlaefer DH, Gifford AT. Cystic endometrial hyperplasia, pseudo-placentation endometrial hyperplasia, and other cystic conditions of the canine and feline uterus. Theriogenology. 2008 Aug;70(3):349-58. 11. Smith FO: Canine pyometra. Theriogenology 66: 610-612, 2006