Signalment:
Gross Description:
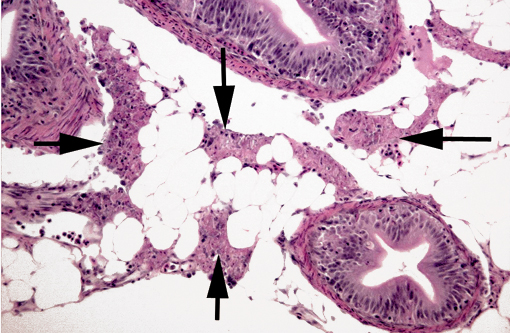
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
IPN is a highly contagious disease of usually acute onset that only affects young fish (<6 months), especially fry; older fish can be infected but are chronic carriers(4). It can present, as in our case, as a sudden and rapid increase in mortality with minimal clinical signs. When present, clinical signs include dorsal darkening, trailing white feces, abdominal distension, exophtalmos, hemorrhages on the ventrum, pale gills and corkscrew spiral swimming4. Mortality is variable, but it is most rapid and severe at temperatures between 10-14oC(4); it can approach 100%. There is no treatment, but fasting and lowering water temperature can diminish the mortality. IPVN infection is often limited to a carrier state; infection is lifelong in carriers and in fish that survive the disease. Many of these fish chronically shed the virus. IPVN can be transmitted vertically and horizontally. It has marked tropism for the exocrine pancreatic epithelium, but IPVN has been demonstrated in kidney, liver and occasionally in other organs in post-smolt Atlantic salmon (Salmo salar L.)(1). Findings of an in vitro study showed that IPNV induced apoptosis followed by postapoptotic necrosis in fish embryonic cells(2). In vivo, IPNV has been shown to induce apoptosis in hepatocytes of post-smolt Atlantic salmon(6); in the exocrine pancreatic epithelium, apoptosis has not been demonstrated.
Gross lesions in fry, when present, include pale viscera with a few petechiae, and a mucoid plug in the GI tract(4). The most characteristic microscopic lesion of IPN is, as its name implies, exocrine pancreatic necrosis(3,5,6), with or without secondary degenerative changes in the adjacent adipose tissue. Other lesions that can be observed are apoptosis of pyloric cecal epithelium (McKnight cells), hepatocyte single cell necrosis/apoptosis, focal myocyte degeneration/necrosis and, in severe cases, necrosis of renal tubular and interstitial cells(4,5). Hepatic lesions have been shown to play a major role in IPN in post-smolt Atlantic salmon(5). A presumptive diagnosis can be established by histopathology but must be confirmed by virological methods (e.g. virus isolation; RT-PCR). Virus isolation in itself is not confirmatory as IPNV can be demonstrated in clinically healthy/carrier fish; thus, a high titer should be present to establish a definitive diagnosis(4). Immunohistochemistry can also be used, and has the advantage of antigen localization. Other viruses can cause exocrine pancreatic necrosis in salmonids; the main differential diagnoses for the lesions in our case are sleeping disease in rainbow trout (Oncorhynchus my kiss) and pancreas disease in salmon, both caused by closely related alphaviruses (Togaviridae)(8). However, in both cases the skeletal and cardiac muscles are also main targets, with significant necrosis, and pancreatic involvement is minimal in sleeping disease(8).
JPC Diagnosis:
1. Exocrine pancreas: Necrosis, multifocal to coalescing.
2. Intestine: Enteritis, necrotizing, multifocal, mild.
Conference Comment:
Conference participants considered the pyknosis and loss of cellular detail in some sections of liver to be autolysis. Conference participants also discussed the epithelial lifting in the secondary lamellae of the gills, which is likely an artifact of formalin fixation rather than edema.
References:
2. Hong JR, Lin TL, Hsu YL, Wu JL: Apoptosis precedes necrosis of fish cell line with infectious pancreatic necrosis virus infection. Virology. 250(1):76-84. 1998
3. Lumsden JS: Gastrointestinal tract, swimbladder, pancreas and peritoneum. In Systemic Pathology of Fish: A Text and Atlas of Normal Tissues in Teleosts and Their Responses in Disease, 2nd ed., pp.168-199. Scotian Press, London, UK, 2006
4. Noga, EJ: Infectious pancreatic necrosis (problem 75). In Fish Disease: Diagnosis and Treatment, 1st ed., pp.208-211. Iowa State University Press, Ames, IA, 2000
5. Noguera PA, Bruno DW: Liver involvement in post-smolt Atlantic salmon, Salmo salar L., infected with infectious pancreatic necrosis virus (IPNV): a retrospective histopathological study. J Fish Dis. 33(10):819-32, 2010
6. Santi N, Sandtr+�-+ A, Sindre H, Song H, Hong JR, Thu B, Wu JL, Vakharia VN, Evensen +�-�: Infectious pancreatic necrosis virus induces apoptosis in vitro and in vivo independent of VP5 expression. Virology. 342(1):13-25, 2005
7. Smail DA, Munro ALS. The virology of teleosts. In: Roberts RJ, ed. Fish Pathology. 3rd ed. Edinburgh, Scotland: Saunders; 2003:211-5.
8. Turnbull, J: Musculoskeletal system. In Systemic Pathology of Fish: A Text and Atlas of Normal Tissues in Teleosts and Their Responses in Disease, 2nd ed., pp.288-320. Scotian Press, London, UK, 2006