Signalment:
Histopathologic Description:
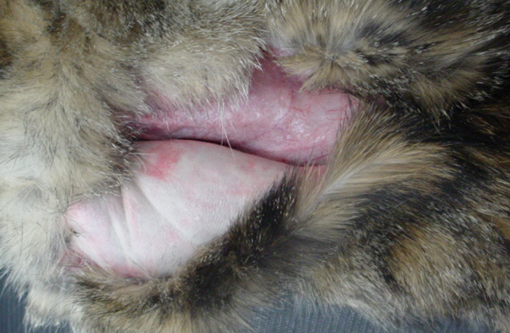
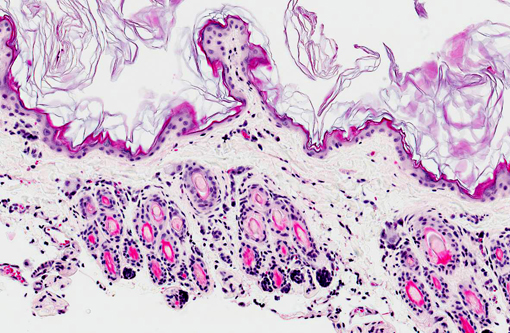
Skin: Two sections of haired skin are examined. The sections consist of epidermis and dermis, with normal appearing hair follicles. The epidermis is thin and ranges from 1-2 cell layers thick, and has diffuse mild orthokeratotic hyperkeratosis. The dermis is severely atrophied and there is loss of a majority of the dermal collagen. The remnant collagen is loosely packed and interspersed with small to moderate numbers of lymphocytes, plasma cells and mast cells. The erector pili muscles within the specimen are prominent.Â
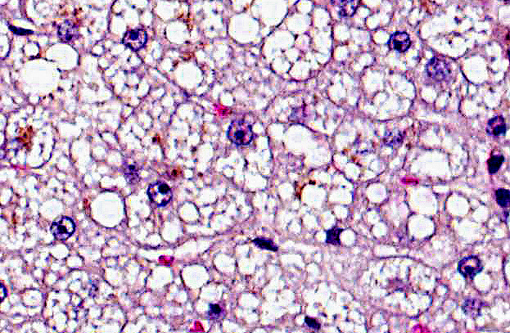
Liver: The cytoplasm of nearly all of the hepatocytes is expanded by several small, distinct, clear cytoplasmic vacuoles (lipid). The swollen hepatocytes have compression of the associated sinusoids. Moderate numbers of hepatocytes, often within the periacinar region have intracytoplasmic green-brown pigment (hemosiderin).Â
Morphologic Diagnosis:
1. Dermal atrophy, diffuse, chronic, severe.
2. Hepatic lipidosis, diffuse chronic, moderate to severe.
Lab Results:
CBC
| Parameter | Value | Reference Range/ Units |
| RBC | 7.5 | 6.54-12.20 M/μL |
| HCT | 37.1 | 30.3-52.3% |
| HGB | 11.4 | 9.8-16.2 g/dL |
| MCV | 49.5 | 35.9-53.1 fL |
| MCH | 15.2 | 11.8-17.3 pg |
| MCHC | 30.7 | 28.1-35.8 g/dL |
| RDW | 20.9 | 15-27% |
| % Retic | 0 | % |
| Retic | 2.3 | 3-50 K/μL |
| WBC | 38.22 | 2.87-17.02 K/μL |
| %Neu | 53.8 | % |
| %Lym | 37.2 | % |
| %Mono | 7 | % |
| %Eos | 2 | % |
| Neu | 20.53 | 1.15-10.29 K/μL |
| Lym | 14.23 | 0.92-6.88 K/μL |
| Mono | 2.69 | 0.02-0.67 K/μL |
| Eos | 0.77 | 0.17-1.57 K/μL |
| Plt | 116 | 151-600 K/μL |
| FeLV | negative | |
| FIV | negative | |
| Heartworm | negative |
Chemistry
| Parameter | Value | Reference Range/ Units |
| GLU | 120 | 74-159 mg/dL |
| BUN | 31 | 15-36 mg/dL |
| CREA | 1.4 | 0.8-2.4 mg/dl |
| BUN/CREA | 22 | |
| PHOS | 5.9 | 3.1-7.5 mg/dL |
| Ca | 9.9 | 7.8-11.3 mg/dL |
| TP | 7.5 | 5.7-8.9 g/dL |
| ALB | 3.4 | 2.2-4.0 g/dL |
| Glob | 4.1 | 2.8-5.1 g/dL |
| Alb/Glob | 0.8 | |
| ALT | <10 | 12-130 U/L |
| ALKP | <10 | 14-111 |
| GGT | 4 | 0-1 U/L |
| TBIL | 0.6 | 0.0-0.9 mg/dL |
| CHOL | 97 | 65-225 mg/dL |
| AMYL | 487 | 500-1500 U/L |
| LIPA | 998 | 100-1400 |
| Na | 169 | 150-165 mmol/L |
| K | 5.6 | 3.5-5.8 mmol/L |
| Na/K | 30 | |
| Cl | 125 | 112-129 mmol/L |
| Osm Calc | 342 | mmol/kg |
| TT4 | 0.8 | μg/dL |
Urinalysis (collected off counter)
| Color | bright yellow | |
| Appearance | clear | |
| SpGr (refractometer) | >1.050 |
Test strip
| Urobilinogen | normal |
| Glucose | negative |
| Ketone | negative |
| Bilirubin | negative |
| Protein | 100 mg/dl |
| Nitrite | negative |
| Leukocytes | 3+ |
| Blood | negative |
| pH | 6.5 on strip, 7.4 on meter |
| Specific gravity | 1.02 |
Sediment
| WBC | 1-2/hpf |
| RBC | rare |
| Epithelial cells | occasional squamous |
| Casts | Waxy (one seen) |
| Crystals | triple phosphate 1-2+ |
| Bacteria | cocci 2+ |
Condition:
Contributor Comment:
Ehlers-Danlos syndrome was considered to be one of the differential diagnoses by the submitting veterinarian. Ehlers-Danlos syndrome was ruled out due to the severe dermal atrophy, being more consistent with acquired skin fragility syndrome. Dermis is of normal thickness and lacks attenuation of dermal collagen seen in Ehlers-Danlos syndrome.(2,5) Another case of dermal atrophy in a cat reports the occurrence after treatment of the cat with phenytoin, but no mechanism was established.(1) It is important to note, phenytoin is metabolized in the liver. Not all cases of FSFS are related to endocrine or hepatic disease. There is a report of fragile skin due to cutaneous histoplasmosis.(7) In that case, the epidermis was attenuated and there was dermal edema. Fibrinoid necrosis of blood vessels in the subcutis with granulomatous inflammation was reported.
Skin fragility cases have been reported associated with a variety of concurrent diseases. However, the mechanism for this syndrome has yet to be determined. Clinical cases of FSFS should be an indication of concurrent disease and further evaluation of cases should be performed.
JPC Diagnosis:
1. Haired skin: Epidermal, dermal, and follicular atrophy, diffuse, severe.
2. Liver, hepatocytes: Lipidosis, diffuse, severe.
Conference Comment:
Conference participants went on to discuss the differential diagnosis for the histological skin lesions. Ehlers-Danlos syndrome is reported (albeit rarely) in cats, and results in hyperextensible skin; however, severe, diffuse attenuation of dermal collagen is not a characteristic feature. Additionally, acquired feline skin fragility syndrome (FSFS) typically presents in middle aged to older cats, while Ehlers-Danlos occurs in young animals.(4) Electron microscopy (not performed in this case) is also a useful tool in diagnosis of Ehlers-Danlos syndrome, which classically demonstrates enlarged, fragmented collagen fibrils.(3) Similarly, ultrastructural studies in cats with FSFS reveal disorganized, tangled, variably sized collagen fibrils, although fibrils are not generally fractured.(4) Paraneoplastic alopecia secondary to pancreatic/biliary carcinoma was also suggested as a possible rule-out. While this condition is associated with follicular atrophy, the dermis is unaffected and epidermal hyperplasia, rather than atrophy, is the most frequent microscopic characteristic; furthermore, the footpads are often involved and skin fragility is not reported, which aids in differentiating this condition from FSFS.(9)
References:
2. Butler WF. Fragility of skin in a cat. Rsch Vet Sci. 1975;19:213-216.
3. Cheville NF. Ultrastructural Pathology: The Comparative Cellular Basis of Disease. 2nd ed. Ames, IA: Wiley-Blackwell; 2009;300.
4. Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Skin Diseases of the Dog and Cat: Clinical and Histopathologic Diagnosis. 2nd ed. Blackwell Science Ltd, Oxford, UK, 2005:389-391.
5. Patterson DF, Minor RR. Hereditary fragility and hyperextensibility of the skin of cats. A defect in collagen fibrillogenesis. Lab Invest. 1977;37:170-179.
6. Stalker MJ, Hayes MA. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol 2. 5th ed. Philadelphia, PA: Elsevier Limited; 2007:310-315.
7. Tamulevicus AM, Harkin K, Janardhan K, Debey BM. Disseminated histoplasmosis accompanied by cutaneous fragility in a cat. J Am Anim Hosp Assoc. 2011;47:e36-41.
8. Trotman TK, Mauldin E, Hoffmann V, Del Piero F, Hess RS. Skin fragility syndrome in a cat with feline infectious peritonitis and hepatic lipidosis. Vet Dermatol. 2007;18:365-369.
9. Turek MM. Cutaneous paraneoplastic syndromes n dogs and cats: a review of the literature. Vet Dermatol. 2003;14:279-296.