WSC 2023-2024, Conference 16, Case 4
Signalment:
9-year-old, male castrated domestic shorthair cat (Felis catus)
History:
The cat presented for a 12-hour history of lethargy and inappetence. Complete blood chemistry revealed severe normocytic normochromic anemia and severe thrombocytopenia. Serum chemistry showed moderate elevation in ALT and mild azotemia. Abdominal radiographs revealed a moderate peritoneal effusion, a suspected small intestinal linear foreign body-associated mechanical obstruction, and generalized hepatomegaly. AFAST and abdominal fluid sampling and cytology confirmed a hemoabdomen of unknown origin. The patient was taken to surgery for an exploratory laparotomy.
Gross Pathology:
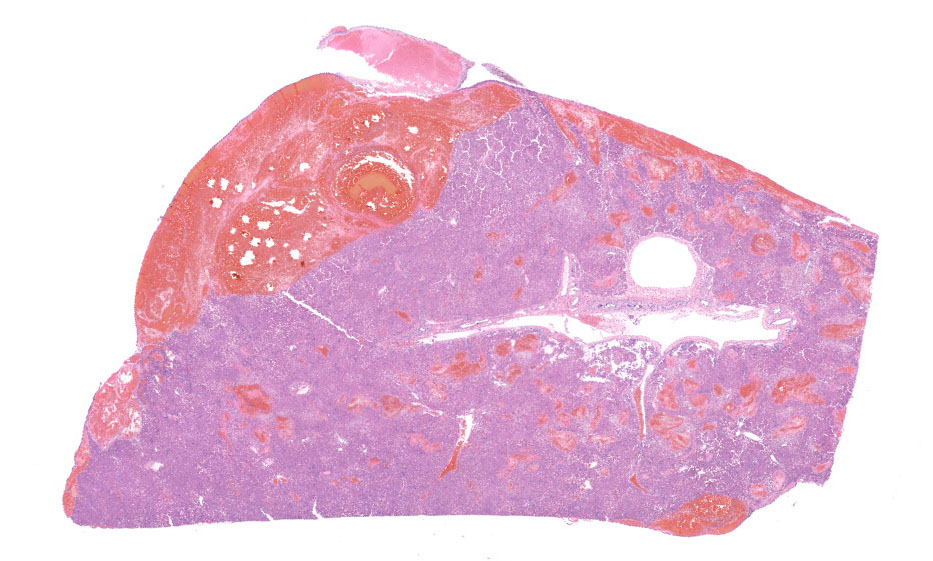
A hepatic mass involving the left and quadrate lobes was observed during exploratory laparotomy. The remainder of the liver parenchyma was mildly friable. No other gross abnormalities were noted during the surgical procedure. The mass was excised and submitted for histopathology.
Laboratory Results:
|
|
Result |
Reference |
Units |
|
WBC |
12.19 |
5.5-19.5 |
x103/ul |
|
Neutrophil |
7.07 |
2.5-12.5 |
x103/ul |
|
Band Neutrophil |
0.00 |
0.0-0.3 |
x103/ul |
|
Lymphocyte |
5.12 |
1.5-7.0 |
x103/ul |
|
Monocyte |
0.00 |
0.0-0.85 |
x103/ul |
|
Eosinophil |
0.00 |
0.0-0.75 |
x103/ul |
|
Basophils |
0.00 |
0.0-0.1 |
|
|
RBC |
3.84 |
4.6-10.2 |
x106/ul |
|
Hemoglobin |
5.5 |
8.5-15.3 |
gm/dl |
|
Hematocrit |
16.5 |
26.0 - 47 .0 |
% |
|
MCV |
43.0 |
38.0 – 54.0 |
fl |
|
MCH |
14.4 |
11.8-18.0 |
pg |
|
MCHC |
33.5 |
29.0-36.0 |
gm/dl |
|
RDW |
19.5 |
16.0-23.0 |
% |
|
Nucleated RBC |
0.00 |
|
|
|
Platelet-Auto |
52 |
100-518 |
x103/ul |
|
MPV |
11.8 |
9.9-16.3 |
fl |
|
|
Result |
Reference |
Units |
|
Albumin |
3.8 |
2.2-4.4 |
gm/dl |
|
Alk Phos |
12 |
10-90 |
IU/L |
|
ALT |
465 |
20-100 |
IU/L |
|
Amylase |
1124 |
300-1100 |
|
|
T. bilirubin |
0.3 |
0.1-0.6 |
mg/dl |
|
BUN |
46 |
10-30 |
mg/dl |
|
Calcium |
10.9 |
8.0-11.8 |
mg/dl |
|
Phosphorus |
3.2 |
3.0-6.9 |
mg/dl |
|
Creatinine |
2.6 |
0.8-2.1 |
mg/dl |
|
Glucose |
87 |
70-150 |
mg/dl |
|
Sodium |
159 |
142-164 |
mEq/L |
|
Potassium |
4.3 |
3.7-5.8 |
mEq/L |
|
T. Protein |
7.5 |
5.4-8.5 |
gm/dl |
|
PTT |
239 |
80-119 |
sec |
Microscopic Description:
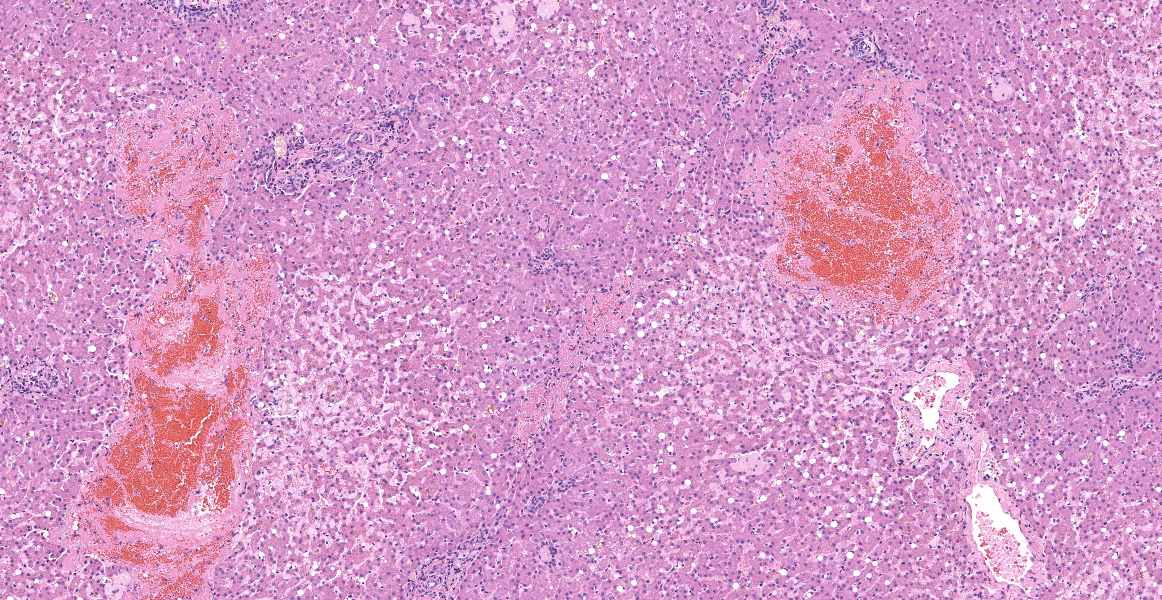
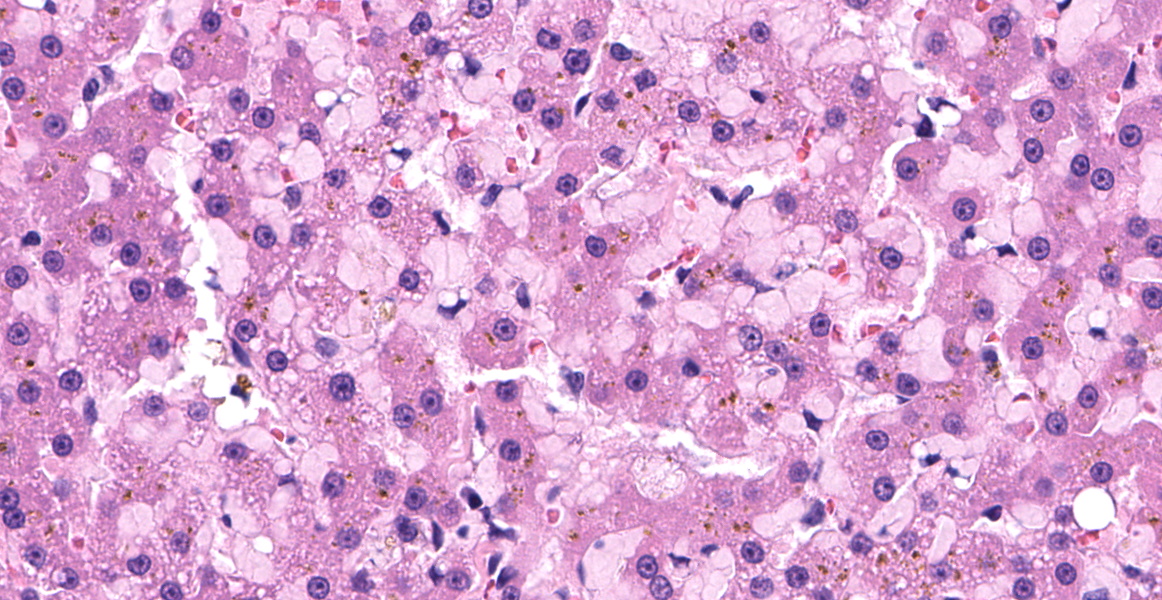
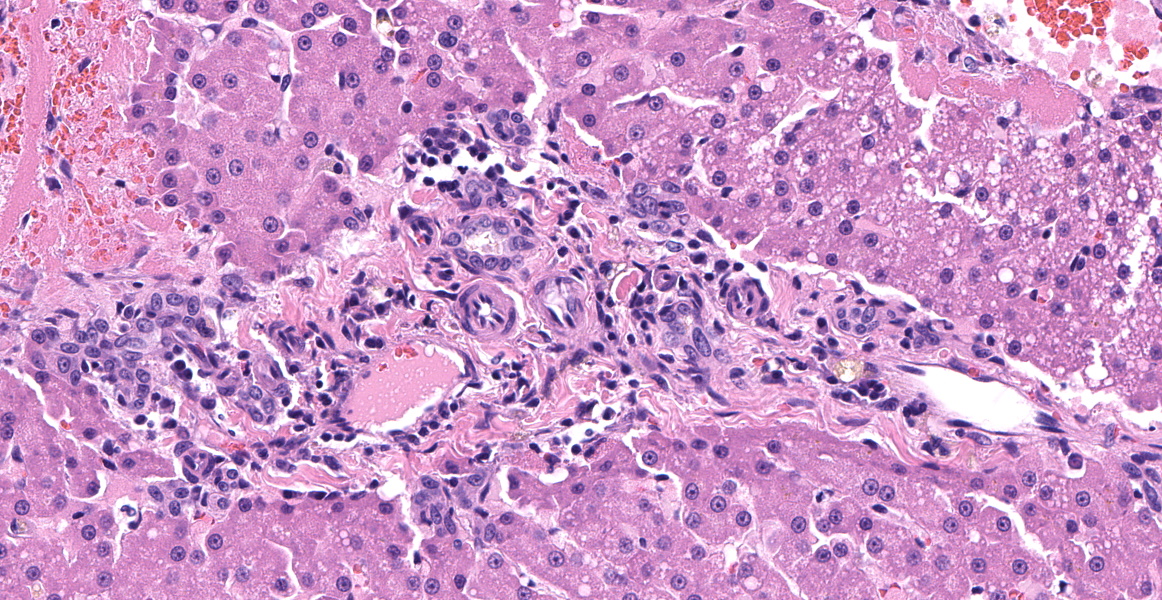
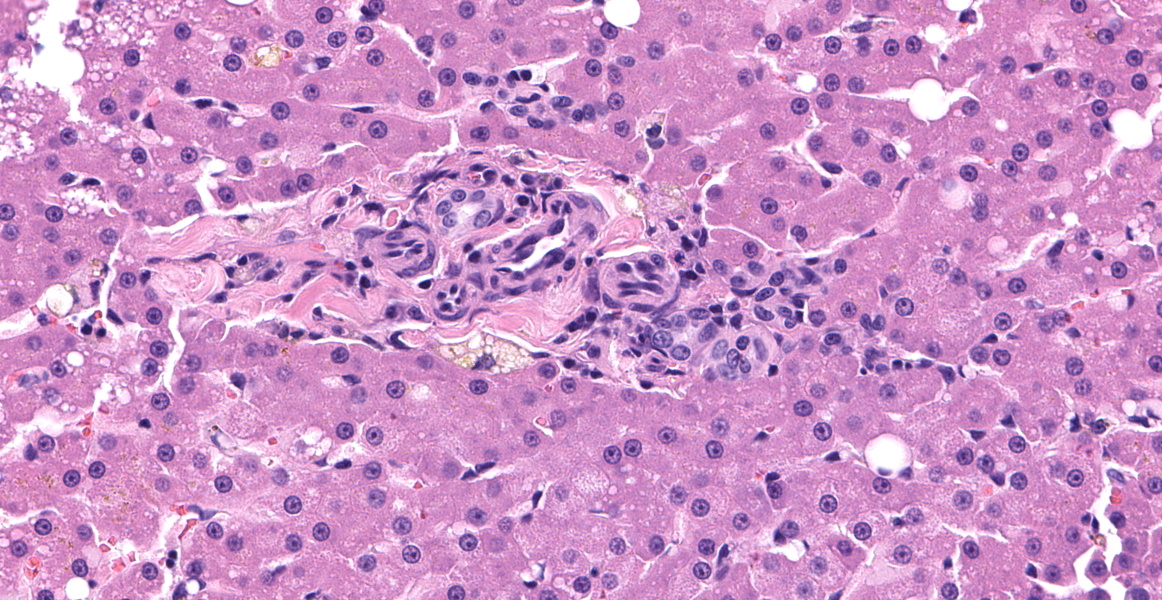
Left lateral liver lobe: Multifocally, portal tracts contain profiles of small arterioles, several of which lack portal venules. Several small arterioles extend into adjacent hepatic parenchyma, and these typically are in a bizarre orientation and have hypertrophied endothelium. Central veins are difficult to identify. The space of Disse in the centrilobular areas contains a substantial amount of eosinophilic, homogeneous, hyaline-like material. Also, there are aggregates of hyaline-like material associated with clusters of cells within the sinusoids. Often, the portal tracts are moderately expanded by infiltration of lymphocytes, plasma cells, and a few eosinophils. There are large foci of disrupted hepatic cords with sinusoids expanded by hemorrhage (telangiectasia), aforementioned hyaline-like material, and fibrin thrombi throughout the parenchyma.
Contributor’s Morphologic Diagnosis:
Left lateral liver lobe:
- Hepatic microvascular dysplasia with portal venule hypoplasia
- Hepatic amyloidosis, with telangiectasia, hemorrhage, and thrombosis
Contributor’s Comment:
This case had morphological findings consistent with hepatic microvascular dysplasia and portal venule hypoplasia as well as amyloidosis that was confirmed by Congo Red staining. The coexistence of these two findings in this patient is considered to be unique.
Hepatic microvascular dysplasia (HMD) is an intrahepatic shunting of blood through microscopic vessels within the liver.3 Clinical signs described in canine patients include inhibited growth, ammonium biurate crystalluria, hepatic encephalopathy, vomiting, and diarrhea.3,7 HMD may occur as an isolated congenital malformation or may be present with a concurrent macroscopic portosystemic shunt vessel.7 Some dogs lacking other hepatic abnormalities, such as a macroscopic congenital portosystemic shunt, may be asymptomatic.7 Rarely, multiple acquired portosystemic shunts may arise secondary to HMD and subsequent portal hypertension.7,12
HMD is fairly frequently reported in small-breed dogs, particularly the Cairn Terrier and Yorkshire Terrier.14 In Cairn Terriers an autosomal recessive pattern of inheritance has been described.11 Cases of HMD and/or portal vein hypoplasia in the cat are infrequently reported in the literature.12 Recently, a case of multiple acquired portosystemic shunts secondary to portal vein hypoplasia was reported in a cat.12,14
Histologic lesions consistent with HMD include increased arterioles in portal triad regions, ectatic pericentral vascular spaces, and mural hypertrophy of central veins.4,7 HMD and portal vein hypoplasia are both congenital defects in the development of intrahepatic portal veins and may occur concurrently.14 Histologically, portal vein hypoplasia is characterized by decreased numbers of portal vein branches within small portal triads.14 If histology reveals evidence of HMD or portal vein hypoplasia, a macroscopic congenital portosystemic shunt must be ruled out by advanced imaging techniques as this abnormality results in similar histologic lesions.7,14
Hepatic amyloidosis is commonly found in association with generalized amyloidosis in the majority of species. Generalized, or systemic, amyloidosis is frequently associated with an overproduction of an amino-terminal fragment of the inducible acute-phase protein serum amyloid A, simply termed “amyloid A.”4 This disease occasionally occurs in a variety of species including horses, cattle, dogs, and cats, and is often a subsequent response to chronic tissue destruction or disease.1,2,4,6,9
Familial amyloid A amyloidosis is another potential underlying etiology of systemic amyloidosis. This disease is recognized in Chinese Shar-Pei dogs and Abyssinian and Siamese cats.8,10 The condition bears a resemblance to familial amyloidosis in humans (familial Mediterranean fever), and affected animals exhibit episodic pyrexia, swollen hocks, renal amyloidosis, and occasional concurrent hepatic amyloidosis. 8,10System
ic amyloidosis in horses is most often secondary to chronic inflammation and is observed with increased frequency in horses used for the production of hyperimmunized serum.1 Affected horses may develop icterus and other signs of hepatic dysfunction.1 In contrast, cattle, dogs, and cats generally first exhibit clinical signs of renal dysfunction from concurrent renal amyloid deposition.6 It is reported that cattle may rather die of the underlying primary disease.6 Though infrequent, cats have been reported to present with spontaneous hepatic rupture, as in this case. 2,6
Contributing Institution:
Iowa State University
College of Veterinary Medicine
Department of Veterinary Pathology
Ames, IA 50010-1250
https://vetmed.iastate.edu/vpath
JPC Diagnoses:
1. Liver: Amyloidosis, intrasinusoidal, multifocal, marked, with parenchymal rupture and acute hemorrhage.
2. Liver: Primary portal vein hypoplasia with numerous portal arteriolar profiles.
JPC Comment:
This beautiful slide, striking at subgross, reveals even more delights upon closer examination. As the contributor nicely describes, the liver is characterized by three major pathologic processes: large accumulations of blood throughout the hepatic parenchyma, multifocal accumulations of eosinophilic hyaline material, and portal areas comprised of increased numbers of arterioles, bile ductules, and portal veins.
On subgross evaluation, the large lakes of hemorrhage are most eye-catching, and conference discussion focused initially on the character of these lesions. Participants wondered if these could represent peliosis hepatis, defined as randomly distributed, cystic blood-filled spaces in the liver.5 This is well-reported in cats and can result either from obstruction of the portal vasculature with subsequent hepatic atrophy and dilation of the sinuisoids (referred to as telangiectasis) or from hepatocyte necrosis.5 Telangiectasis is distinguished from hemorrhage by the presence of an endothelial lining, and participants discussed which process was predominant in this lesion, decided that the lakes of blood represented hemorrhage as they were not lined by discernable endothelium.
Discussion then turned to the eosinophilic deposits, interpreted by the contributor and conference participants as amyloid.
A Congo Red performed at JPC in preparation for the conference was negative, prompting considerations of other differentials for the the eosinophilic material; however, in cats, amyloid is often best illustrated using thioflavin T staining and a post-conference thioflavin T staining performed at the University of California, Davis, was weakly positive, confirming the material as amyloid.
Discussion of the portal abnormalities quickly turned semantic, with participants discussing the terms “microvascular dysplasia” versus “portal vein hypoplasia.” This distinction is challenging and likely artificial due to the stereotypical histologic response of the liver to inadequate portal vein flow.5 With decreased portal blood flow, the portal vein and its tributaries become reduced and hepatic arteries respond to this hypoperfusion by increasing their blood flow, in the processes becoming more tortuous, proliferative, and hypertrophic.5 Histologically, as seen in this case, this is evidenced by an increased number of arteriolar profiles in the portal tracts, sometimes with an accompanying increase in the number of biliary ductular profiles along with slight fibrosis.5 The increased arterial hepatic flow may result in increased sinusoidal pressure and subsequent sinusoidal dilation.5
Primary portal vein hypoplasia is a congenital disorder of dogs and, rarely, of cats, which is characterized by the histologic findings typical of portal vein hypoperfusion discussed above.5 The World Small Animal Veterinary Association’s standardization board for canine and feline liver circulatory disorders has stated that there have been “no clinical or biochemical findings to suggest that [microvascular dysplasia] is different from primary portal vein hypoplasia” and that the authors “have decided to abandon the name microvascular dysplasia” and prefer to call this condition “primary portal vein hypoplasia,” which the authors find more descriptive.5
Primary portal vein hypoplasia varies widely in clinical severity and morphology and overlaps histologically with other causes of hypoperfusion, including congenital portosystemic shunts, intrahepatic arterioportal fistulas, and portal vein obstruction.5 In 30% of dogs, the vascular changes are mild with no evidence of portal fibrosis; others have moderate to marked fibrosis in the portal tracts, hypoplasia or absence of the portal veins, and a proliferation of arterioles and bile ductules, as seen in this case.5 Importantly, this diagnosis is based on histologic examination of a liver biopsy in combination with ultrasonographic findings which exclude the presence of congenital portosystemic shunt, arteriovenous fistula, or portal vein thrombosis.5 In this case, the portal areas contain all the classic hallmarks of hypoperfusion; however, the case history contains no record of ultrasonographic findings to exclude the other causes of hypoperfusion.
Examination of a reticulin stain illustrated complete destruction of the reticulin framwork in this case. Dr. Pesavento noted that no research currently exists on whether this is a feature of microvascular dysplasia or amyloid deposition, but the reticulin destruction in this case was striking, with small remnant fragments of reticulin extending only a short distance from central veins. The lack of a reticulin framework and the hemorrhagic presentation in this case is reminiscent of fatty liver hemorhagic syndrome in poultry, in which vacuolar swelling of hepatocytes disrupts the reticulin structure of the hepatic cords, leading to hemorrhage from the sinusoids.13
Given that the principal cause of death was hemorrhage, presumably due to hepatic fracture secondary to amyloidosis, participants favored placing amyloidosis front and center in the morphologic diagnosis. Despite the persistence of the term “microvascular dysplasia” in the literature, participants preferred to use the WSAVA-recommended term “primary portal vein hypoplasia” to capture the full complement of architectural derangements present in the examined portal areas.
References:
- Abdelkader SV, Gudding R, Nordstoga K. Clinical chemical constituents in relation to liver amyloidosis in serum-producing horses. J Comp Pathol. 1991;105(2):201-211.
- Blunden AS, Smith KC. Generalised amyloidosis and acute liver haemorrhage in four cats. Vol 5. J Small Anim Pract. 1992;33(12):566-570.
- Christiansen JS, Hottinger HA, Allen L, Phillips L, Aronson LR. Hepatic microvascular dysplasia in dogs: a retrospective study of 24 cases (1987-1995). J Am Anim Hosp Assoc. 2000;36(5):385-389.
- Cullen J, Stalker M. Liver and Biliary System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Vol 2. Elsevier 2016:278-279.
- Cullen MJ, van den Ingh TSGAM, Bunch WE, Rothuizen J, Washabau RJ, Desmet VJ. Morphological classification of circulatory disorders of the canine and feline liver. In: Rothuizen J, ed. WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Disease. Elsevier;2006:41-59.
- Elitok OM, Elitok B, Unver O. Renal amyloidosis in cattle with inflammatory diseases. J Vet Intern Med. 2008;22(2):450-455.
- Hwang T, An S, Kim A, Han C, Huh C, Lee HC. Acquired portosystemic shunts secondary to hepatic microvascular dysplasia in a young dog. J Vet Clin. 2020; 37(2):88-90.
- van der Linde-Sipman JS, Niewold TA, Tooten PC, de Neijs-Backer M, Gruys E. Generalized AA-amyloidosis in Siamese and Oriental cats. Vet Immunol and Immunopathol. 1997;56(1-2):1-10.
- Neo-suzuki S, Mineshige T, Kamiie J, et al. Hepatic AA amyloidosis in a cat: cytologic and histologic identification of AA amyloid in macrophages. 2017;46(2):331-336.
- Olsson M, Meadows JRS, Truvé K, et al. A novel unstable duplication upstream of HAS2 predisposes to a breed-defining skin phenotype and a periodic fever syndrome in Chinese Shar-Pei dogs. 2011; 7(3):e1001332.
- Schermerhorn T, Center SA, Dykes NL, et al. Characterization of hepatoportal microvascular dysplasia in a kindred of cairn terriers. J Vet Intern Med. 1996;10(4):219-230.
- Sugimoto S, Maeda S, Tsuboi M, et al. Multiple acquired portosystemic shunts secondary to primary hypoplasia of the portal vein in a cat. Vol. 80, J Vet Med Sci. 2018:874-877.
- Trott KA, Giannitti F, Rimoldi G, et al. Fatty liver hemorrhagic syndrome in the backyard chicken: a retrospective histopathologic case series. Vet Pathol. 2015; 51(4):787-795.
- Watson P. Canine breed-specific hepatopathies. Vet Clin North Am Small Anim Pract. 2017;47(3):665-682.