Signalment:
4-year-old, female Anglo Nubian goats (
Capra hircus).
Four out of twenty-five stud goats from a herd in Northern NSW died suddenly after displaying ataxia, tachycardia, dilated pupils, pyrexia (38.2C), head tilt and stargazing. The submitting clinician necropsied one animal, and found a diffuse white pattern in the liver. No other changes were reported. The entire brain and sections of liver were submitted. There was a recent introduction of new hay.
Histopathologic Description:
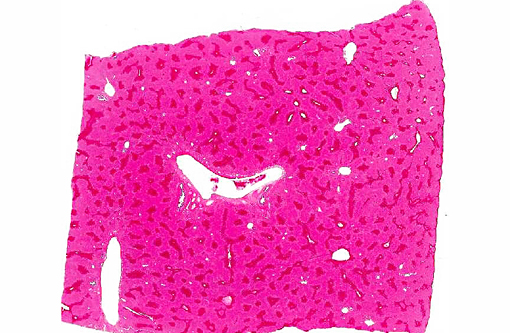
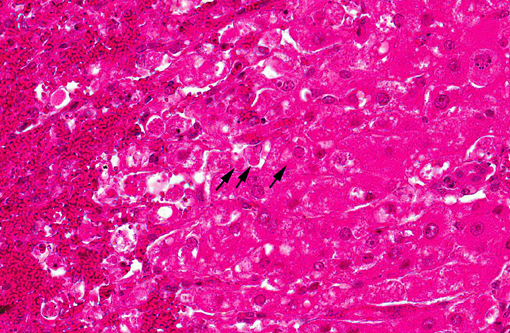
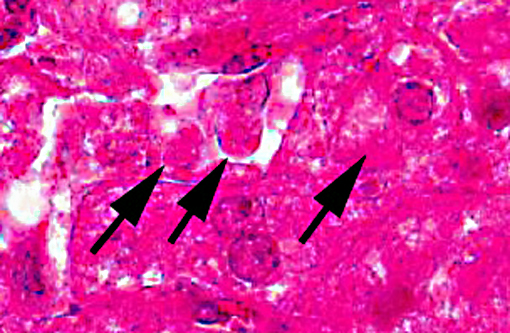
There is extensive centrilobular necrosis with replacement hemorrhage. Hepatocytes at the margins of the necrotic zones contain multiple fine vacuoles. Occasional cytosegresomes are noted, and individualized, shrunken hepatocytes are scattered through the section. There is mild to focally moderate hepatocytic cellular size variation, with an increase in mitotic figures. Larger bile ducts are frequently lined by hyperplastic epithelium, and there are multiple zones with increased numbers of intraepithelial lymphocytes.Â
Morphologic Diagnosis:
1. Hepatic necrosis, centrilobular, severe, coalescing, acute.Â
2. Mild biliary epithelial hyperplasia.Â
Condition:
Trema tomentosa (poison peach) toxicity
Contributor Comment:
The submitting veterinarian made a note that a number of potential poisonous plants were present in the fields, including a heavy growth of
Trema tomentosa (formerly
Trema aspera), more commonly known as poison peach.Â
Poison peach is a large leafy shrub or small tree distributed along Eastern and Northern Australia, extending into New Guinea. It grows mainly in moist open forest, and is considered a pioneer species frequently becoming a dominant growth following deforestation. Cattle and sheep readily eat the leaves when access is given. In Australia, leaves and fine stems have been shown to be toxic to many livestock species - presumably due to the presence of a glycoside named trematoxin, which acts as a hepatic toxin.(2) Death due to its ingestion has been reported in Australia in cattle, sheep, horses and camels. Experimentally, toxicity can be easily induced in rabbits and guinea pigs. The submitters have been unable to find any description of toxicity in the literature.
Its relative,
Trema micrantha, is widely dispersed in Central and South America. It is also a pioneer species and is being considered as a prime candidate in replantings and restoration of degraded forest land. The tree contains toxins which induce a profound hypoglycaemia in rats, and some trials have been carried out touting this agent as an alternative treatment for diabetes mellitus in humans.(4) One reference (in Portuguese) was found citing toxicity in cattle(4) and experimentally in rabbits(6), both of which suggest pathological changes similar, if not identical, to those seen in
Trema tomentosa. Toxicity in livestock induced by
T. micrantha appears to be more prevalent in Brazil, explaining the tendency for more recent reports to be in the non-English literature.Â
The differential diagnosis for this histological pattern in ruminants should include the common suspects of microcystin (blue green algae), green cestrum (
Cestrum spp.), and noogoora burr (
Xanthium pungens). The chinaberry or white cedar tree (
Melia azedarach) contains four teranortriterpenes (meliatoxins A1, A2, B1 and B2), which can variably lead to massive central necrosis; however, these changes are less uniform that those noted above.(3)
JPC Diagnosis:
Liver, centrilobular hepatocytes: Degeneration and necrosis, diffuse, acute, with marked hemorrhage.
Conference Comment:
We have seen several examples of various types of hepatic toxicity in this years conferences. The liver is a common site of toxic injury, as it is exposed to numerous ingested substances through the portal blood. There are six categories of hepatotoxic liver injury, based on the cellular targets of the injurious substances. The most common mechanism involves biotransformation by the cytochrome p-450 system that results in toxic metabolites. Because cytochrome p-450 is most abundant in the centrilobular areas, the result is centrilobular necrosis, as in this case of poison peach toxicity. The second category is characterized by the formation of adducts between drugs and cellular enzymes, other proteins or nucleic acids that act as antigens that, once recognized by the immune system, trigger an inflammatory response against the hepatocytes or biliary epithelium that contain them. A third category involves toxins (such as retained or excess bile acids) that trigger apoptosis of hepatocytes either by direct stimulation of pro-apoptotic pathways or immune mediated events that release TNF-alpha or activate Fas apoptosis pathways. The fourth category of hepatic toxicity is caused by the disruption of calcium homeostasis by cell membrane damage or disruption of enzymes responsible for maintaining intracellular calcium concentrations. This disruption leads to increased intracellular calcium, which activates proteases that damage actin filaments such that further damage to cell membranes results. The fifth category of toxic injury to the liver manifests as intracellular cholestasis, as the pumps that secrete bile into the canaliculi can be disrupted by certain chemicals (such as estrogen, erythromycin) or by hepatocellular injury that affects the canalicular pumps or actin filaments around the canaliculi. Finally, the sixth category of hepatic toxicity is hepatocellular injury and death due to mitochondrial damage. Mitochondrial damage can result in hepatocyte injury, apoptosis or necrosis via several mechanism, including release of reactive oxygen species, decreased ATP, and decreased beta-oxidation of lipids leading to microvesicular steatosis (lipid accumulation), or release of cytochrome-c which triggers apoptosis.(1)
The distribution of hepatocellular injury reflects the mechanism and cellular targets of the toxin. Toxins that require bioactivation by cytochrome p-450 metabolism result in centrilobular or paracentral (periacinar) degeneration and necrosis. Midzonal degeneration and necrosis are uncommon lesions, but have been reported with aflotaxicosis in pigs and horses. Periportal hepatocellular injury occurs with cholestasis-related injury or toxins that do not require cytochrome p-450 metabolism (e.g. white phosphorous).(1) Other mechanisms, such as toxins that cause mitochondrial or cellular membrane damage, may result in a more diffuse distribution of injury.Â
References:
1. Cullen JM, Brown DL. Hepatobiliary system and exocrine pancreas. In: McGavin MD, Zachary JF, eds.Â
Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier Mosby; 2012:437-439.
2. Oelrichs PB. Isolation and purification of trematoxin from
Trema aspera.Â
Phytochemistry. 1968:7:1691-1693.
3. Oelrichs PB, Hill MW, Vallely PJ, et al. Toxic tetranortriterpenes of the fruit of
Melia azedarach.Â
Phytochemistry. 1983;22:531-534.
4. Schoenfelder T, Cirimbelli TM, Citadini-Zanette V. Acute effect of
Trema micrantha (
Ulmaceae) on serum glucose levels in normal and diabetic rats.Â
Journal of Ethnopharmacology.Â
2006;107:456-459.
5. Traverso SD, Correa AMR, Schmitz M, et al. Experimental poisoning by
Trema micrantha (Ulmaceae) in cattle.Â
Pesquisa Veterinaria Brasileira. 2004;24:211-216.
6. Traverso SD, Driemeier D. Experimental
Trema micrantha (Ulmaceae) poisoning in rabbits.Â
Veterinary and Human Toxicology. 2000;42:301-302.