CASE II: H20-1916-5 (JPC 4159332)
Signalment:
A 1-year-old ram lamb (Ovis aries)
History:
Animal was presented for necropsy following a brief clinical history of 2 to 3 days of scours, and a 4-day history of anorexia and hyperdipsia. The producer noted that the lamb often used a provided salt lick.
Gross Pathology:
The lamb was in good body condition. The right cranial and middle lung lobes were consolidated, with yellow-tinged foam exuding from small airways on the cut surface. The abomasal mucosa was grey, with few scattered petechiae.
Laboratory Results:
· Fecal flotation (sugar): Trichostrongylus sp., light parasite load. Coccidian parasite of unknown identity, light parasite load.
· Ileocecal valve culture: E. coli, coagulase negative Staphylococcus sp.
· Lung culture: E. coli, coagulase negative Staphylococcus sp., Gram negative bacilli of unknown identity.
Microscopic Description:
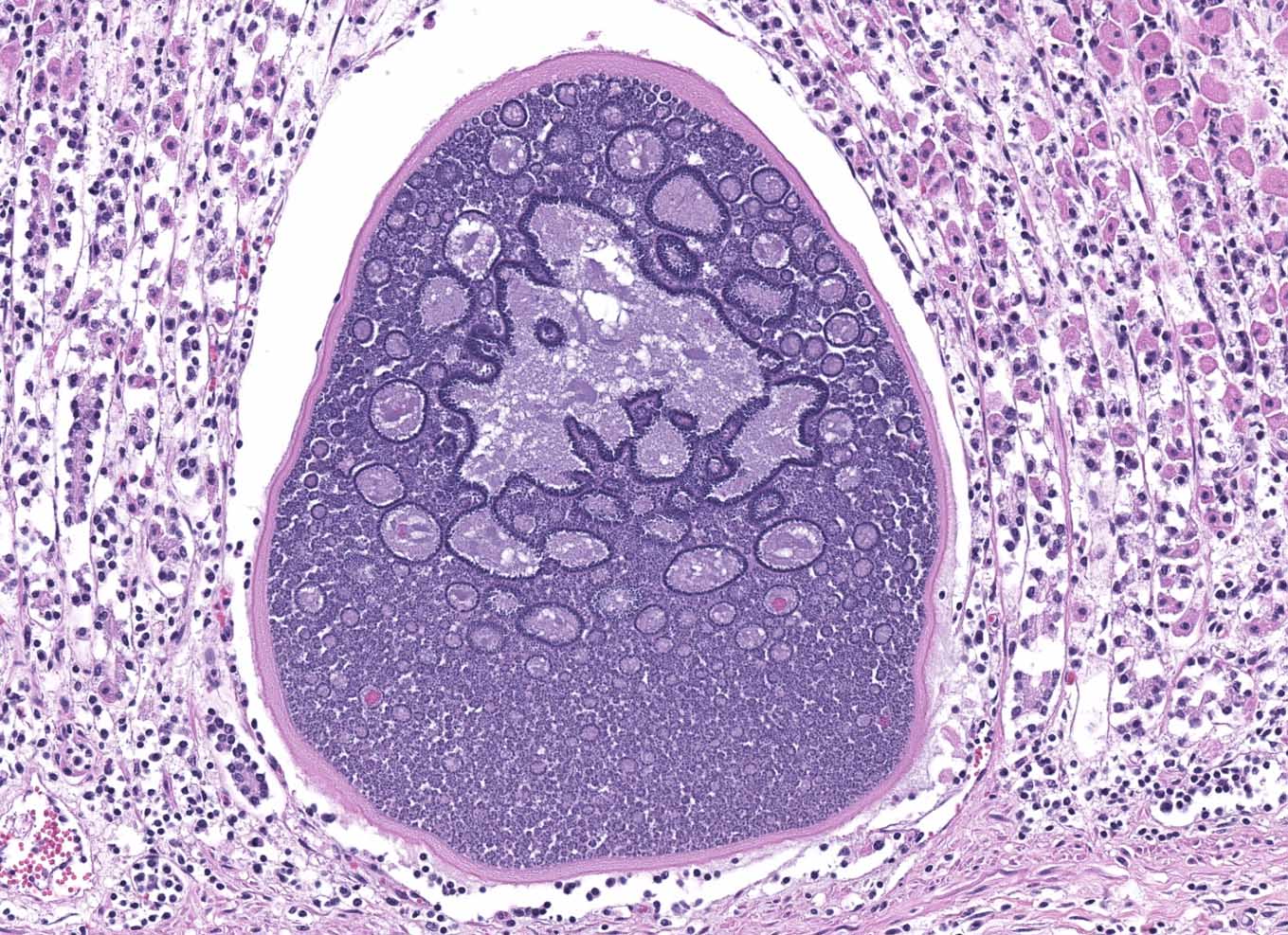
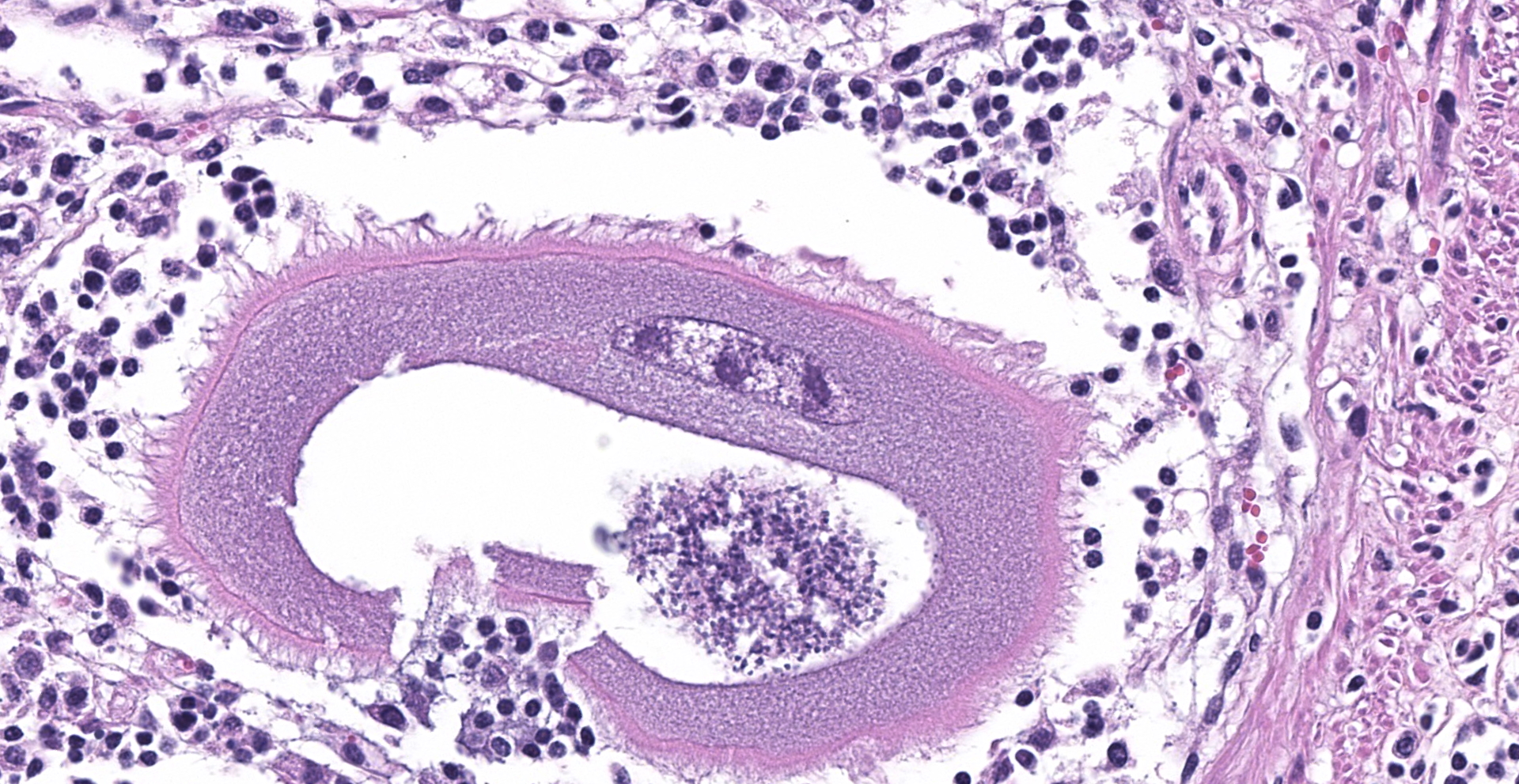
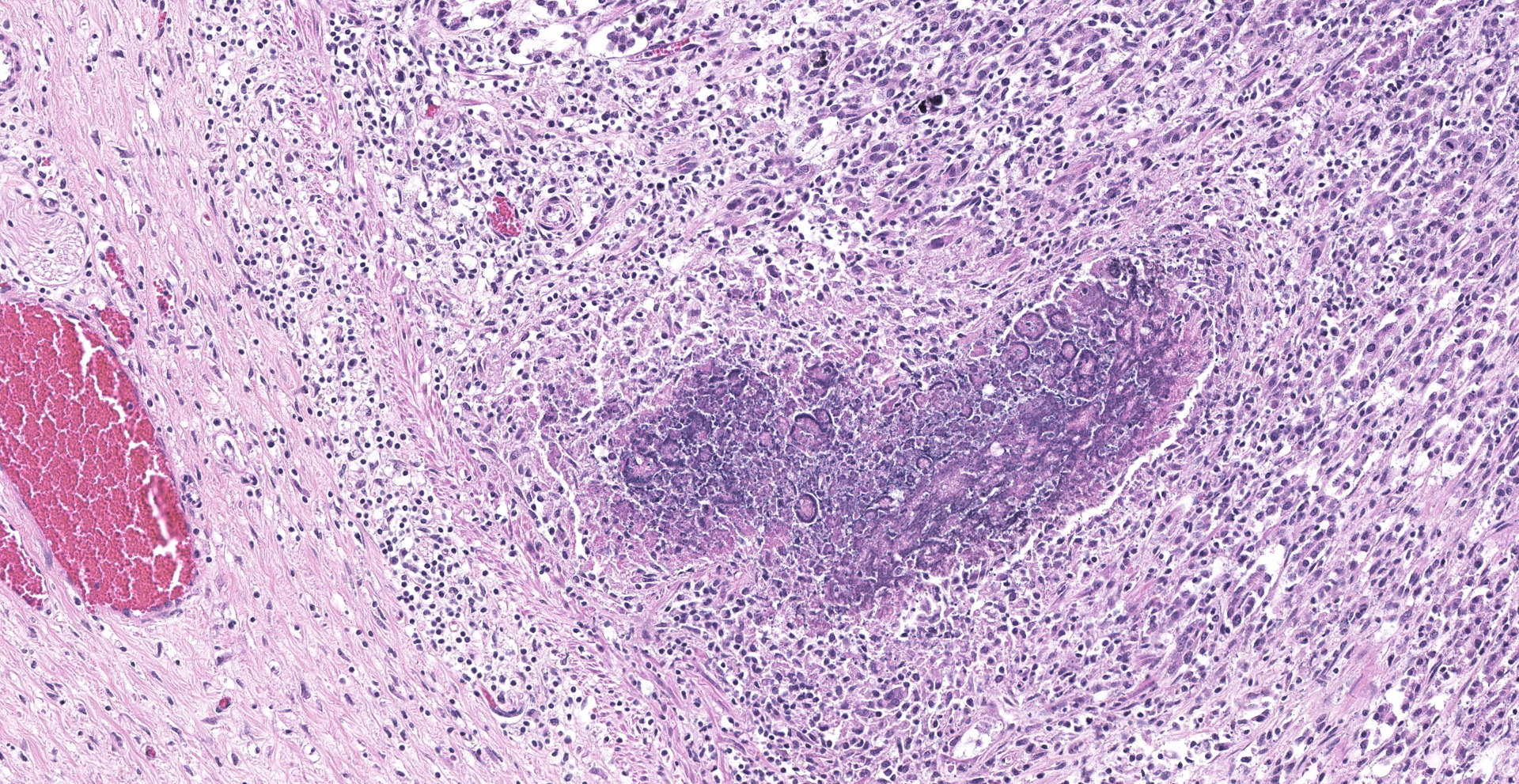
Abomasum: Multifocally within the mucosa, there are multiple cysts of apicomplexan parasites that measure 20-250 µm diameter, have a thick capsule (schizonts) and contain numerous merozoites frequently arranged in blastophores. Some of these cysts are ruptured, with focal areas of necrosis of the mucosa plus infiltrates of large numbers of neutrophils, macrophages, and occasional multinucleated giant cells, which are phagocytizing merozoites. The lamina propria and the submucosa are infiltrated with moderate numbers of lymphocytes, plasma cells and macrophages. In the mucosa, there are a few fibrin thrombi.
Contributor's Morphologic Diagnosis:
Abomasum: Abomasitis, neutrophilic and histiocytic to granulomatous, multifocal, with mucosal necrosis and numerous intralesional apicomplexan parasites.
Contributor's Comment:
The morphology of the abomasal parasite in this case is consistent with the apicomplexan parasite Eimeria gilruthi, an incompletely characterized coccidian parasite of the gastrointestinal system in sheep, which was most recently described in the Journal of Veterinary Diagnostic Investigation in 2019.1 These coccidia have a characteristic microscopic morphology, with large protozoal schizonts ("megaloschizonts") with a thick, eosinophilic wall surrounding thousands of elongate merozoites, often arranged in circular blastophores. Megaloschizonts can be so large that they form miliary, pale, pinpoint foci on the mucosal surface of the abomasum visible on postmortem examination. 4
Although many species of Eimeria can be found within the alimentary tract of sheep, few are considered pathogenic. E. crandallis and E. ovinoidalis are the two paramount pathogenic species, both of which typically affect lambs up to 6 months old, especially those reared under crowded or stressful conditions. A recent diagnosis of E. gilruthi in a group of 15-month-old ewes demonstrated its pathogenicity of this coccidian parasite even in older animals.1 Clinical signs are typical of many intestinal parasites and usually consist of progressive anemia, diarrhea, anorexia, and weakness. Rapid weight loss in production animals often leads to euthanasia, although the protozoan itself can cause lethal disease in severe cases. Damage to host cells is typically through traumatic rupture following the growth and expulsion of merozoites and, for many Eimeria species, the severity of clinical disease correlates with the parasite burden.2
This diagnostic exercise would be remiss if it did not mention the life cycle of this parasite. Although the life cycle of E. gilruthi is not completely elucidated, it very likely mirrors that of other Eimeria species.2 A typical life cycle of these species includes both a sexual and an asexual form of reproduction. The infectious stage is the sporozoite, which is capable of penetrating and infecting host intestinal cells, and which typically enters the gastrointestinal system via ingestion by the host of sporulated oocysts from the environment. Once intracellular, the sporozoites undergo a morphological change into trophozoites, which are located within a membrane-bound parasitophorous vacuole within the host cell cytoplasm.2 The trophozoites further expand into structures known as schizonts, which produce merozoites through asexual reproduction. In E. gilruthi in particular, these schizonts develop to such a massive size that they are called megaloschizonts.2,4 Merozoites exit the cell (typically in a manner that damages the host cells) and infect neighboring host cells. There is a finite number of asexual reproductive cycles possessed by each Eimeria species, which differs between the species; the number is not yet elucidated for E. gilruthi. After the final asexual reproductive cycle, the resulting merozoites develop into either a microgamont (male sex cell) or macrogamont (female sex cell). The macrogamont further develops by growing in size and storing energy; the fully mature female sex cell is a macrogamete.2 Microgamonts undergo repeated nuclear division, eventually splitting into multiple, biflagellate, uninuclear microgametes. A zygote forms when a microgamete penetrates and fertilizes a macrogamete (sexual reproduction); it is referred to as an oocyst when it develops hyaline granules and a wall. The oocyst exits the host cell via rupture of the cell and trafficking through the feces, and sporulates within the environment.2
Contributing Institution:
Department of biomedical Sciences and Pathobiology, Virginia Maryland Regional College of Veterinary medicine, Virginia Tech https://www.vetmed.vt.edu/departments/dbsp/
JPC Diagnosis:
Abomasum: Apicomplexan megalo-schizonts, with mild lymphoplasmacytic and neutrophilic abomasitis.
JPC Comment:
The contributor provides an excellent review of Eimeria gilruthi, an entity making its inaugural appearance in the WSC.
Abomasal lesions consistent with E. gilruthi were first described by Maske in 1893, who erroneously attributed them to gregarines, a group of apicomplexan parasites now known to only infect invertebrates. Similar lesions were later observed by Gilruth and studied in greater detail by Chatton in 1910, who named the organism Gastrocystis gilruthi, although multiple early reports identified it as Globidium giltruthi. E. gilruthi underwent multiple scientific name changes over the ensuing decades with the current nomenclature initially being favored by Levine and Soulsby during the 1960s.3
Interestingly, sheep and goats may not be definitive hosts for E. gilruthi. Despite the apicomplexan's ability to develop one or more generations of schizonts in small ruminants, reports are conflicting in regard to its ability to undergo gametogony. Attempts to close such knowledge gaps are confounded by the parasite's sporadic occurrence and attempts to replicate its life cycle in vitro have so far been unsuccessful.1
Common post-mortem findings associated with E. gilruthi include edema and multifocal raised white foci within the mucosa of the abomasum, which histologically correlate to megalo-schizonts. However, Teladorsagia circumcincta (previously known as Ostertagia circumcincta), a trichostrongyle nematode, may cause similar gross lesions in the abomasum of sheep and goats. Microscopic examination is required for differentiation between these entities.1
The majority of coccidian parasites, including Eimeria spp., are host specific. Notable exceptions include E. pallida, E. caprovina, E. punctate, and E. gilruthi, which have been reported in both sheep and goats.4
The following table identifies hosts and organs affected by coccidian species.4
|
Animal |
Coccidia |
Organ affected/Clinical signs |
|
Birds Chickens
Turkey
Geese & ducks
Sandhill/whooping cranes Parrots |
E. acervulina E. necatrix/maxima E. brunette E. tenella E. meleagridis E. adenoeides E. meleagrimitis E. gallopavonis E. truncata E. anseris/nocens E. reichenowi E. psittaculae |
Duodenum/enteritis Jejunum/enteritis Ileum/enteritis Ceca/typhylitis Cecum Cecum, ileum Upper intestine Ileum, large intestine Kidney/anorexia, depression Intestine Disseminated Intestine |
|
Cattle
|
E. bovis/zuernii E. alabamensis |
Cecum and colon/diarrhea Small intestine |
|
Sheep |
E. ahsata/christenseni E. brakuensis E. crandallis E. ovinoidalis |
SI SI SI Cecum, colon |
|
Goats |
E. christenseni E. arloingi E. hirici E. ninakohlyakimovae |
SI SI SI LI |
|
Equine |
E. leukarti Klossiella equi |
SI |
|
Swine |
E. debliecki |
SI (in 1-3 week old piglets) |
|
Canine |
I. canis |
Ileum, colon occasionally |
|
Feline |
I. felis |
SI, colon occasionally |
|
Mice |
Klossiella muris E. falciformis E. vermiformis E. papillata E. ferrisi |
kidney Colon Intestine Intestine Intestine |
|
Rabbit |
E. stiedae E. intestinalis E. flavescens |
Bile ducts Ileum & cecum Ileum & cecum |
|
Ferret |
E. furonis |
SI |
References:
- Ammar SI, Watson AM, Craig LE, et al. Eimeria gilruthi-associated abomasitis in a group of ewes. J Vet Diagn Invest. 2019;31(1):128-132.
- Bowman DD. Georgis' Parasitology for Veterinarians. 9th ed. St. Louis, MO: Saunders Elsevier; 2009: 93-94.
- Hilali M. Studies on globidial schizonts in the abomasum of Norwegian sheep. The fine structure of one of the four merozoite forms investigated. Acta Vet Scand. 1973;14(1):22-43.
- Uzal, FA, Plattner BL, Hostetter, JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. Vol 2. 6th ed. St. Louis, MO: Elsevier; 2016:54-55, 227-235.