CASE 1: 18/530 (4137582-00)
Signalment: 2 years, female, Norwegian kystgeit, Capra hircus, goat.
History:
The goat was from a small flock of 15 Norwegian kystgeit, which is an old goat breed kept historically for meat production. The flock was kept on a barren pasture due to an unusually dry and warm summer. The goat was examined several times during a period of two weeks. The goat was lethargic and very thin, had poor appetite and was lying down. There were no respiratory or gastrointestinal signs, and the feces had normal consistency. Blood samples showed hypocalcemia, hypomagnesemia, and anemia. The goat was treated with calcium, magnesium, NSAIDs and penicillin.
Gross Pathology:
Subcutaneous tissues on extremities, the body and ventrally on the head were moist due to edema. Mucosal membranes were pale. The chest cavity contained 100 mL transudate, and lungs were congested and edematous with abundant frothy fluid in trachea and bronchi. Epicardial fat had partially gelatinous and moist appearance. The rumen was moderately filled with normal content. The abomasal wall was thickened due to severe submucosal edema.
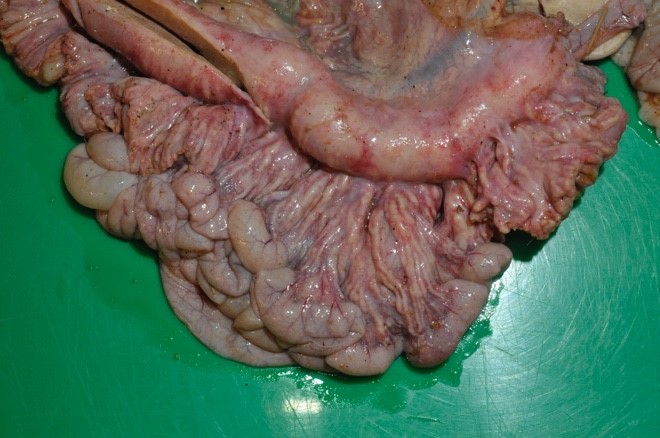
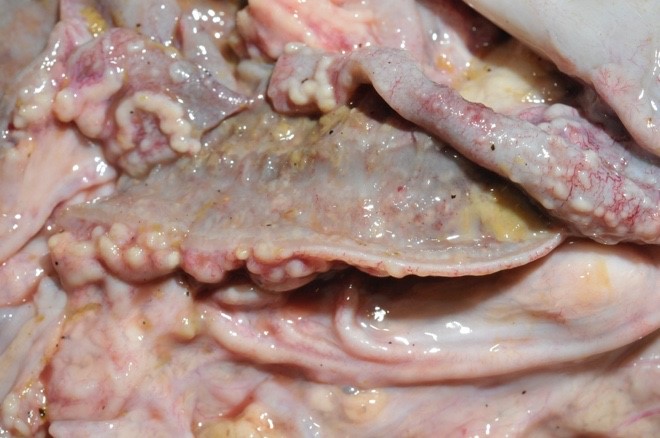
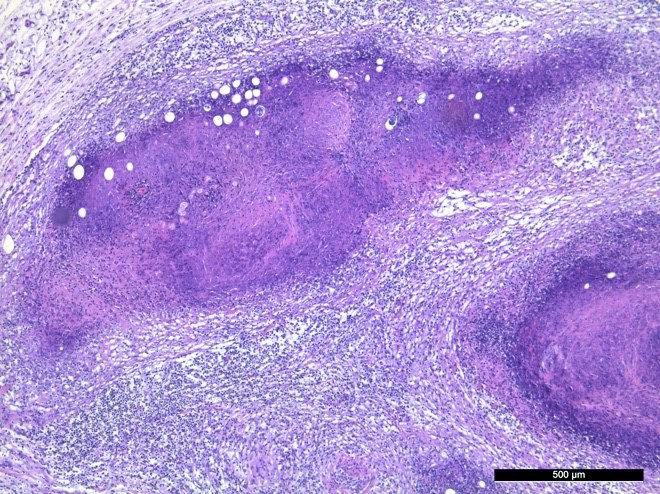
The mesenteric lymph nodes were severely enlarged with caseous necrosis which usually affected the entire cut surface of the nodes. Some nodes had a dark red cut surface with multiple grey to white, up to 0.5 cm large nodules. Mesenterial lymphatics were severely thickened, grey to white in color, and firm.
Severe lesions were present in the small intestine, starting 4 meters aborally to the pylorus, and continuing to and including the ileocecal opening. In the jejunum there were multifocal, up to 0.5 cm large, grey to white nodules in the mucosa. Ileum had a diffusely thickened wall, with a grey to white necrotic mucosa. The aboral small intestine had small amounts of watery and blood tinged content. Nodules in the intestinal wall had a caseous center. The serosa of affected small intestine had multiple small white nodules. In the colon there were sparse amounts of normal intestinal content.
Laboratory results:
On initial bacteriological culture from ileum and mesenterial lymph node, a mixed bacterial flora of Escherichia coli, hemolytic Escherichia coli and Enterococcus sp. were detected. Mycobacteriological culture from small intestinal content and mesenterial lymph node showed growth of Mycobacterium avium subsp. hominissuis. Small intestinal content investigated by real time PCR was positive for the same bacterium. Bacteriological culture and PCR was negative for Mycobacterium avium subsp. paratuberculosis.
Microscopic description:
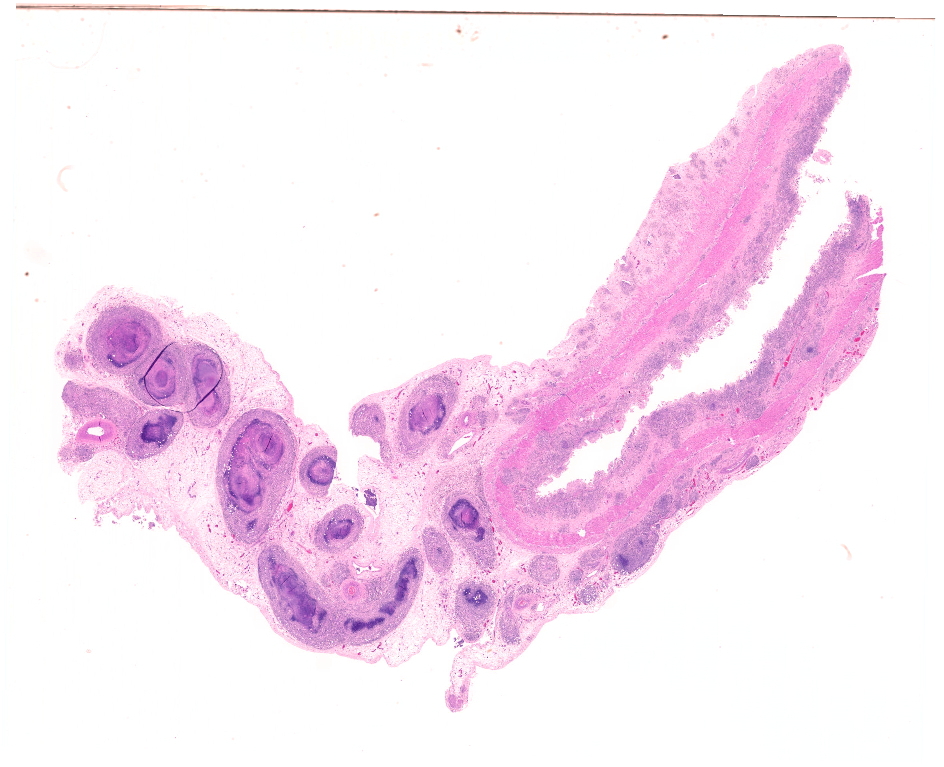
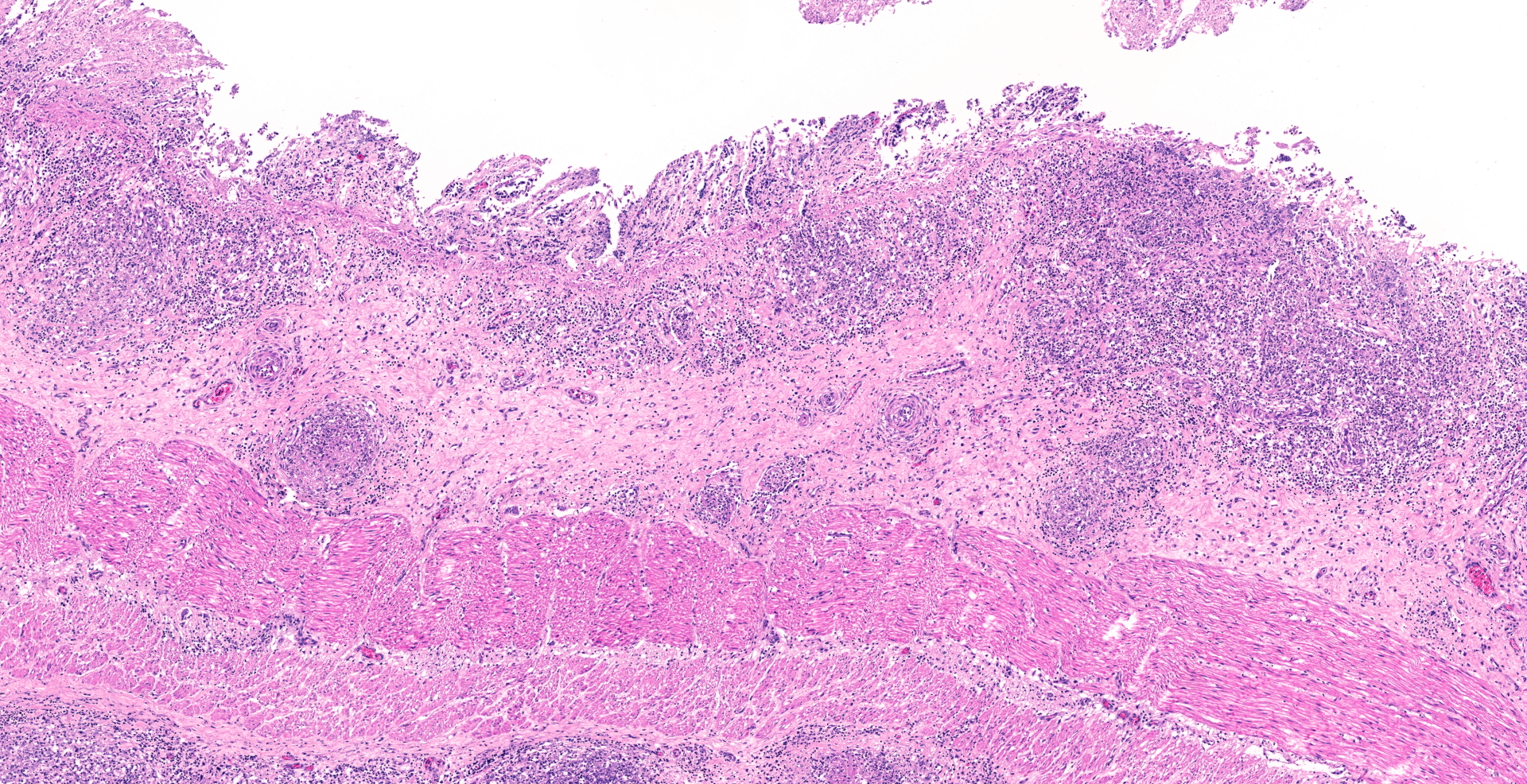
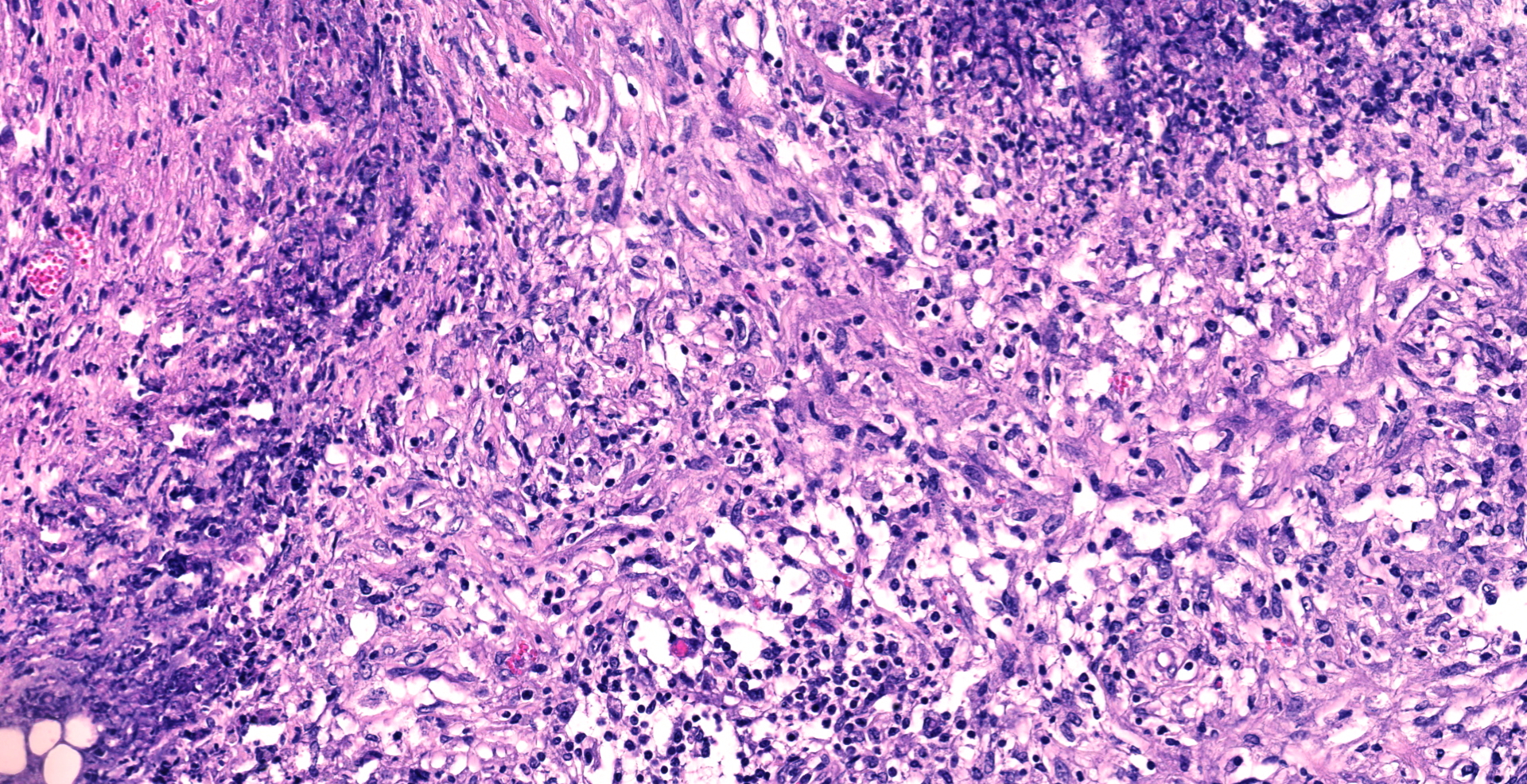
The mucosa of the small intestine is mostly missing, due to a combination of autolytic changes and necrosis. In remnants of mucosa there is a diffuse infiltration of neutrophils and macrophages and some lymphocytes and plasma cells. In the submucosa there are distinct multifocal to confluent areas dominated by macrophages and neutrophils in areas interpreted to be remnants of gut-associated lymphoid tissue, some of which have necrotic centers. Between these, there is a diffuse infiltration of macrophages, neutrophils, lymphocytes and plasma cells.
In the serosa and in the mesentery, there are multiple granulomas with necrotic centers surrounded by a rim of macrophages and some neutrophils, surrounded by infiltration of lymphocytes and plasma cells. With increasing size of the granulomas there are increasing fibroblast proliferation in the periphery. Several granulomas have large vacuoles on the border between the necrotic center and the zone with macrophages and fibrosis. Some granulomas are associated with vascular structures, and areas of inflammation affected lymphatic vessels. Some granulomas have dystrophic mineralization in the center.
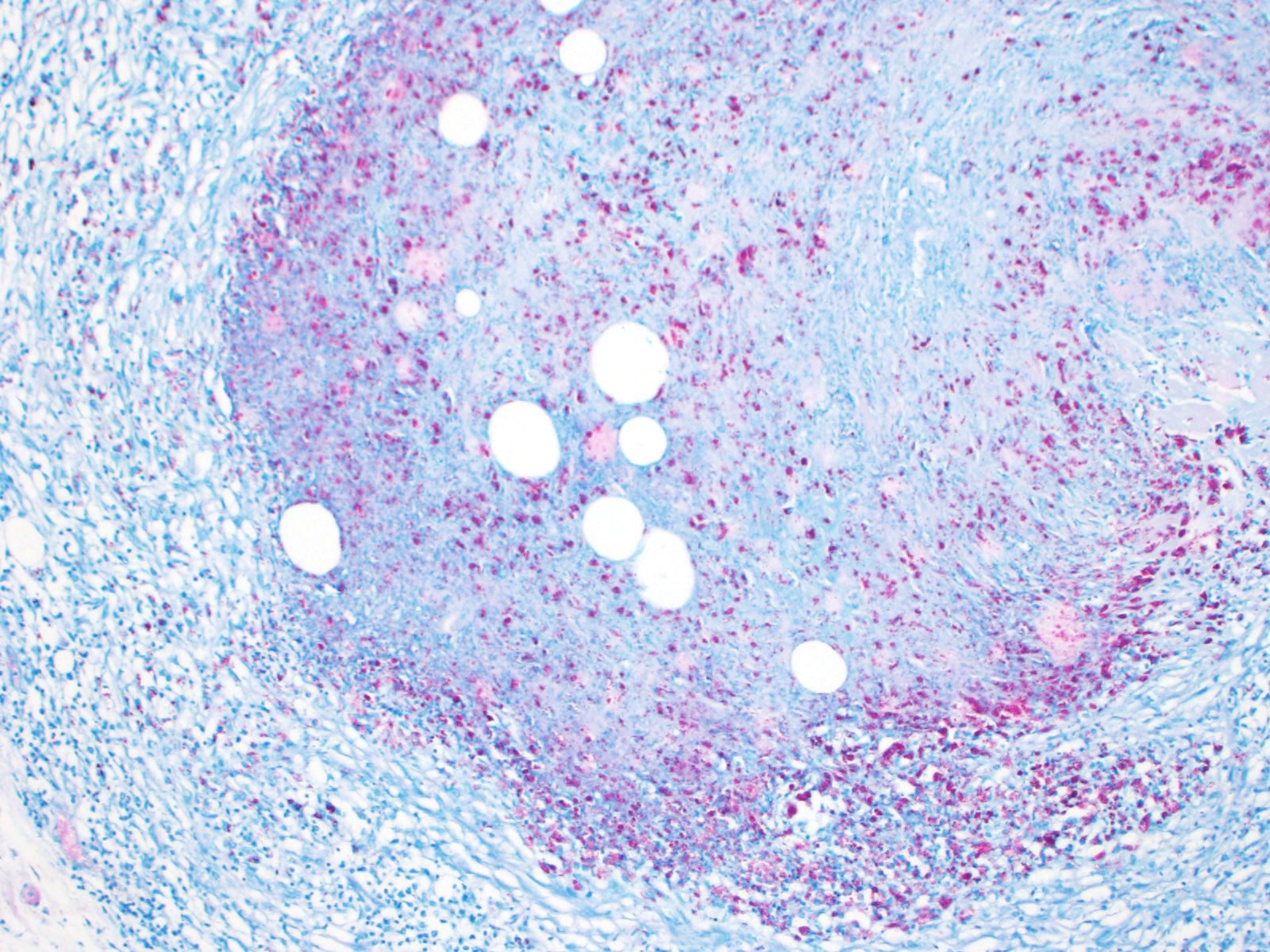
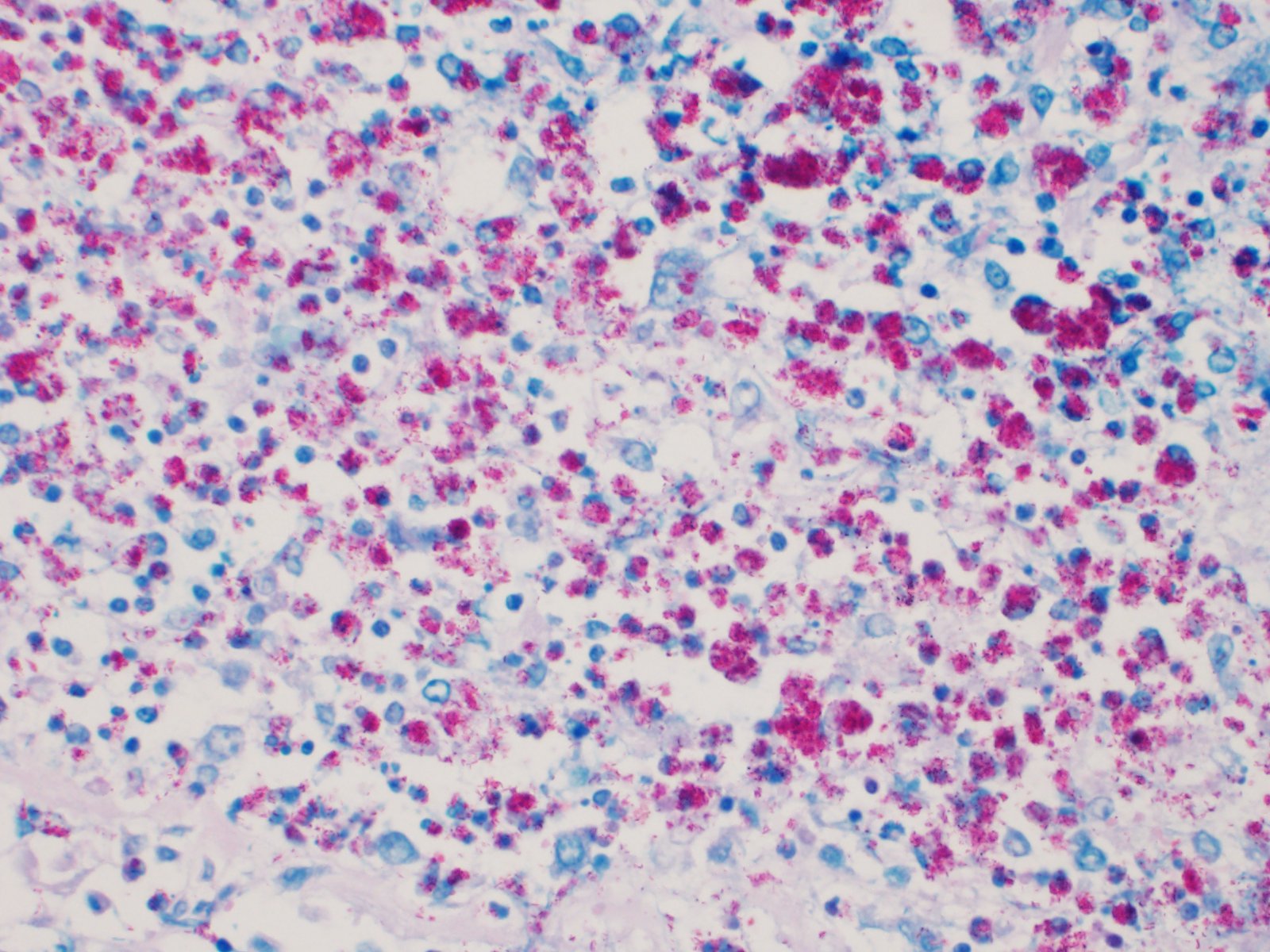
Ziehl-Neelsen stain revealed numerous intracellular acid-fast bacilli in macrophages.
Contributor's morphologic diagnosis:
Small intestine and mesentery: Multifocal to confluent granulomatous enteritis with serosal and mesenterial granulomatous lymphangitis with severe caseous necrosis.
Contributor's comment:
In the Mycobacterium avium complex (MAC), there are 9 species of slow-growing mycobacteria and a further subset of isolates of undetermined classification (the so-called "MAC-others").6 The species Mycobacterium (M.) avium contain four distinct subspecies; M. avium subsp. hominissuis (MAH), M. avium subsp. paratuberculosis (MAP), M. avium subsp. avium (MAA), and M. avium subsp. silvaticum (MAS). Although closely related, theses subspecies represent distinct organisms, each with specific pathogenic and host range characteristics. They range from environmental opportunistic bacteria that cause infections in swine and immunocompromised human patients, to pathogens of birds and ruminants.6 The MAH was quite recently suggested as a separate subspecies, to reflect the distinction of human and porcine isolates from bird-type strains.5
Paratuberculosis, caused by MAP and characterized by chronic wasting in goats, was suspected due to the clinical signs and the gross pathology observed at necropsy. However, culture and PCR were negative for this bacterium and MAH was cultured and detected by PCR.
MAH is an environmental opportunistic pathogen for humans and swine worldwide, but the organism has also been isolated from numerous other animals, such as cattle, dogs, birds, deer and horses.6 MAH has also been shown to be able to cause disease in goats after experimental oral infection, characterized by a large variety of granulomas in organized gut-associated tissues and intestinal lymph nodes. In severely sick goats, 2-3 months post infection, there were granulomas with extensive necrosis, little mineralization and variable numbers of acid-fast bacteria, surrounded by many CD4+ T cells, but only few epithelioid macrophages.7 As the granulomas develop with time, they were characterized with a granuloma stage 1-6, and in the older granulomas there is more extensive caseous necrosis, fewer epithelioid macrophages and multinucleated giant cells, more distinct fibrosis and more lymphocytes,7 characteristics consistent with lesions in the present case. Many of the granulomas in the present case, both in the serosa and the mesentery, had extensive necrosis, but mineralization was only seen in a few granulomas. Two out of three sections from the mesenterial lymph node from the present case had total necrosis of the lymph node, surrounded by a thick zone of connective tissue. The third section of the mesenterial lymph node had severe multifocal to confluent necrosis.
Soil and water are considered to be the natural reservoirs for MAH. Drinking water and tap aerosols are thought to be the main source for human infections, and for swine the source may be drinking water, feed, bedding materials, soil, wastewater, invertebrates and possibly other materials.6
A study of genotypes and strain distribution of MAH isolated from humans and pigs in Belgium revealed a large genetic diversity of strains and an absence of a link between genotypes and the place of residence (human) or the farm or origin (pigs), suggesting an environmental source of infection.8
The flock with the submitted case was held on a barren pasture, due to unusual and severe drought and warm weather in Norway in the summer of 2018. It is possible that the weather and pasture conditions may have contributed to an increased likelihood of an oral infection with MAH, however to the contributor's knowledge, this was the only documented case of MAH induced disease in animals in the country that summer.
Contributing Institution:
Norwegian University of Life Sciences
Faculty of Veterinary Medicine
PO Box 369 Sentrum
0102 Oslo
Norway
JPC diagnosis:
Small intestine, mesentery: Lymphangitis, granulomatous, multifocal to coalescing, chronic, severe, with neutrophilic and histiocytic enteritis, Norwegian kystgeit, caprine.
JPC comment:
There was spirited debate during the conference about the neutrophil content in some of these lesions. While acid fast stains revealed numerous acid-fast bacilli in macrophages as expected, the autolysis of the tissue section may have obscured an additional neutrophilic process.
The contributor summarizes the current classification of Mycobacterium avian spp succinctly. While the discussed four subspecies affect veterinary species, the remaining identified species in "MAC" primarily affect humans (M. intracellulare, M. colombiense, M. chimaera, M. marseillense, M. timonense, M. boucherdurhonense, M. vulneris, M. arosiense, and "MAC-others").1
In contrast to Mycobacterium tuberculosis, Mycobacterium avian sp. express serovar specific glycopeptidolipids (ssGPL), which play a role in inhibiting macrophages activation, and in immunomodulatory activity by downregulating Th1 response. Gene lysX (lysyl-transferase-lysyl-tRNA synthetase) is involved in the lysinylation of surface phospholipids, which makes the bacterium susceptible to antimicrobial peptides. Wild type, mutated, or absent lysX genes affect many aspects of virulence, including rate of macrophage fusion, GPL expression and antigenic reactivity, susceptibility to defensins, and tolerance toward oxidative and nitrosative stress. The patterns of change in these factors is different between MAH and Mycobacterium tuberculosis but provides an interesting area of future research.4
Mycobacterium avium subspecies hominissuis was recently confirmed as the causative agent of disseminated disease in a cat in 2019. The species was confirmed by culture and single nucleotide polymorphism analysis, the first diagnosis of MAH confirmed by gene analysis.2
While MAH rarely causes disease in horses, a complete genome sequence of MAH was completed from the stomach contents of an equine abortion case in Japan in 2019.3
Two typical acid-fast stains are Ziehl-Neelsen and Fite-Faraco, and Mycobacteria spp. exhibit "acid-fastness" due to the high mycolic acid content in their cell walls. All Mycobacterium spp. have this property, but a number of Actinomycetes also have mycolic acid in their cell walls and have some degree of acid-fastness. Notable bacteria include Nocardia spp, and Rhodococcus spp, which are also pathogens of veterinary concern.
While not classified in MAC, Mycobacterium tuberculosis continues to be a problematic pathogen around the world. Noted historical figures Jane Austen, George Orwell, John Keats, Walt Whitman, Paul Gaugin, Frederic Chopin, several kings of France, and Florence Nightingale all suffered and died from tuberculosis. Even more recent figures, such as Ringo Starr experienced infection as a child and subsequently recovered.
In 1882, Robert Koch, a Prussian physician, used a new staining method and used it on sputum of afflicted people. He was the first person to visualize the pathogen Mycobacterium tuberculosis. His research on human tuberculosis was the foundation of his development of the framework known as "Koch's Postulates", which criteria to establish a causal relationship between a microbe and a disease. Sir Bradford Hill built upon Koch's Postulates and established 9 criteria for causation (Bradford Hill criteria) in 1965, which are debated, but often still applicable today.
References:
1. Eslami M, Shafiei M, Ghasemian A, et al. Mycobacterium avium paratuberculosis and Mycobacterium avium complex and related subspecies as causative agents of zoonotic and occupational diseases. Journal of Cellular Physiology. 2019;234(8):12415-12421.
2. Kanegi R, Yasugi M, Nabetani T, et al. Clinical findings and treatment of disseminated Mycobacterium avium subspecies hominissuis infection in a domestic cat. Journal of Veterinary Medical Science. 2019;81(12):1842-1849.
3. Kinoshita Y, Niwa H, Uchida-Fujii E, Nukada T. Complete genome sequence of Mycobacterium avium subsp. hominissuis strain JP-H-1, isolated from an equine abortion case in Japan. Microbiol Resour Announc. 2019;8:e01228-19.
4. Kirubakar G, Schafer H, Rickerts V, Schwarz C, Lwein A. Mutation on lysX from Mycobacteriumavium hominissuis impacts the host-pathogen interaction and virulence phenotype. Virulence. 2020;11(1):132-144.
5. Mijs W, de Haas P, Rossau R, et al. Molecular evidence to support a proposal to reserve the designation Mycobacterium avium subsp. avium for bird-type isolates and 'M. avium subsp. hominissuis' for the human/porcine type of M. avium. Int J Syst Evol Microbiol. 2002;52:1505-1518.
6. Rindi L, Garzelli C. Genetic diversity and phylogeny of Mycobacterium avium. Infect Genet Evol. 2014;21:375-383.
7. Schinköthe J, Köhler H, Liebler-Tenorio EM. Characterization of tuberculous granulomas in different stages of progression and associated tertiary lymphoid tissue in goats experimentally infected with Mycobacterium avium subsp. hominissuis. Comp Immunol Microbiol Infect Dis. 2016;47:41-51.
8. Vluggen C, Soetaert K, Duytschaever L, et al. Genotyping and strain distribution of Mycobacterium avium subspecies hominissuis isolated from humans and pigs in Belgium, 2011-2013. Euro Surveill. 2016;21:30111