Signalment:
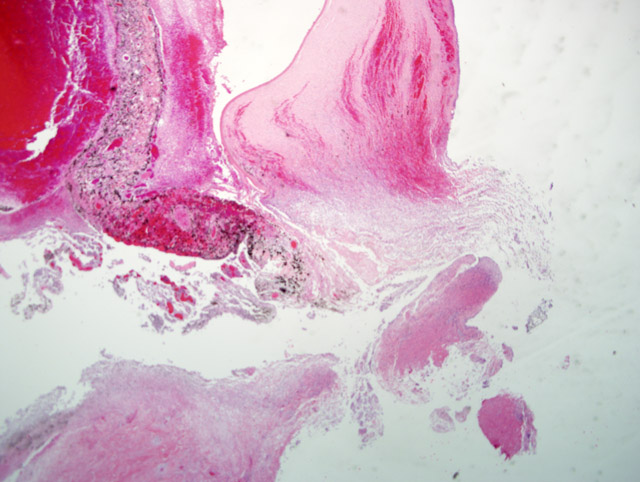
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
The presence of corneal perforation and associated lens inflammation point to the likelihood of a penetrating foreign body. Although the history is vague in this case, and while younger animals are more often affected, a cat claw wound was deemed most likely. These wounds cause (6) corneal and lens capsule perforation with ensuing phacoclastic uveitis. Phthisis or glaucoma resulted in 50% of the cases. Grass awns and porcupine quills are also frequently cited as sources of ocular or orbital foreign bodies in dogs.(2-4,6)
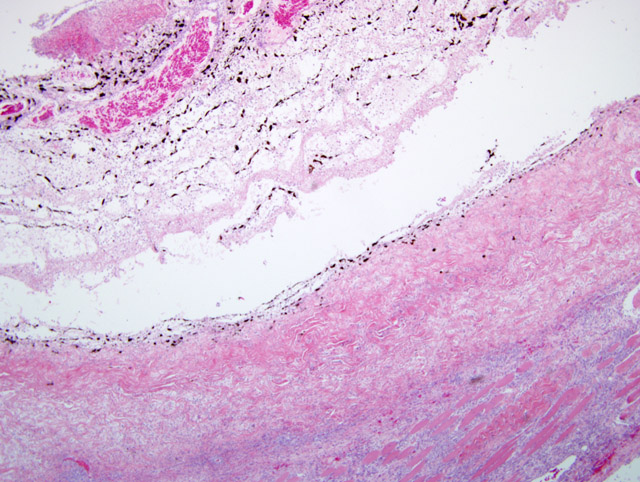
In 20 cases of lens rupture that excluded animals with intractable intraocular infection, lens-induced uveitis was characterized by capsular rupture, cataract, lymphoplasmacytic iridocyclitis, and perilenticular inflammation.(8) Moreover, capsule defects were in line with breaks in the Descemets membrane similar to the one in this case. Even in the absence of infection, the affected lens will become invested with neutrophils, lens epithelium and/or metaplastic fibroblasts. In 13/20 cases in this study, the vitreous and posterior uvea were also inflamed. Four dogs with recent perforations had fibrinopurulent inflammation surrounding the lens.
Phacoclastic uveitis is distinguished from phacolytic uveitis in that the latter is a result of reaction to protein leakage from a cataractous lens and without other associated causes of uveal inflammation.(7) In both conditions, normal low dose tolerance to lens proteins is thought to be overwhelmed by rupture of the lens and release of high dose antigen. When lens lysis occurs, lens membrane proteins are also released, and this results in a more powerful presentation of antigens to the systemic immune system that is likely to overwhelm tolerance.
This case demonstrates the potentially catastrophic results of intraocular inflammation, which had at the time of enucleation involved every structure of the eye. Panophthalmitis, defined as inflammation of all the tunics of the eye, is much less common than endophthalmitis, defined as inflammation of the uvea, retina, and ocular cavities. The acute changes near the site of corneal rupture contrast with those inside the eye. These are of longer duration based on the presence of an anterior fibrovascular membrane, corneal vascularity, and the neovascularization at the back of the eye. Wilcock points out that many cases of traumatic lens laceration are detected by a lack of response to treatment, (8) and in his cases, the interval between injury and uveitis onset (when known) was 4-20 days.
JPC Diagnosis:
Conference Comment:
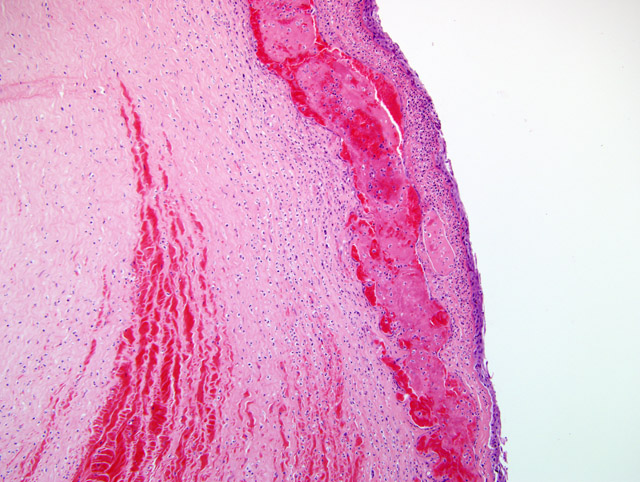
The cornea is avascular and covered by nonkeratinized, nonpigmented stratified squamous epithelium. This thin, non-pigmented epithelium allows for clarity of vision.(9) The stroma also maintains a state of relative dehydration aided by numerous epithelial and endothelial tight junctions in combination with a Na-K-dependent ATP-ase pump in the cell membrane of the corneal endothelium.(9) Bowmans layer is just below the squamous epithelium. Bowmans layer was discussed during the conference because it is a distinctive membrane in humans, but is not distinct in mammals. Underlying Bowmans layer, and compromising 90% of the thickness of the cornea, is the corneal stroma and layers of collagen interspersed with fibroblasts. Descemets membrane, distinct in domestic animals, lies between the corneal stroma and the corneal endothelium.(1)
The cornea is a highly specialized area of the body; therefore, it is protected from damage by numerous mechanisms including an antibacterial tear film and a physically movable barrier known as the eyelid. After damage, the cornea often quickly takes on water. Water enters from the eye's anterior aspect via lacrimal secretions and the posterior aspect via fluid from the anterior chamber. Even more water enters the cornea if the electrolyte pump is compromised because of corneal damage.
If injury to the cornea is superficial and only involves the epithelium, this defect heals by epithelial cells sliding over the defect, followed by mitosis after approximately 24 hours.(9) Chronic ulcers often require additional resources to heal. These resources are drawn from the epithelium of the corneoscleral junction where permanent populations of cells available for replication reside. Cells recruited from the corneoscleral junction tend to retain phenotypic characteristics of conjunctiva including pigmentation and rete ridges. This is an easy way to recognize a chronic ulcer. This is referred to as conjunctival (or cutaneous) metaplasia.
If the underlying stroma of the cornea is damaged in addition to the epithelium, rebuilding of the damaged stroma may be required before epithelial repair can occur. Within a few hours of the initial insult, neutrophils enter the wound and begin to kill bacteria, degrade damaged collagen, and stimulate fibroplasia and vascularization. Stromal cells at the edge of the defect and fibroblasts recruited from the limbus produce new stroma and cover the defect.(9) New blood vessels begin to form approximately 4 days after an extensive injury, and this ingrowth is from the limbus and progresses at a rate of about 1 mm a day. This lag time is important because superficial wounds can heal in less than 4 days without the help of vascularization. If vascularization occurs during healing, visual impairment from stromal fibroplasia is often the result.(9)
References:
2. Brennan KE, Ihrke PJ: Grass awn migration in dogs and cats: A retrospective study of 182 cases. J Amer Vet Med Assoc 182:1201-1204, 1983
3. Bussanich MN, Rootman J: Intraocular foreign body in a dog. Can Vet J 22:207-210, 1981
4. Grahn BH, Szentimrey D, Pharr JW, Farrow CS, Fowler D: Ocular and orbital porcupine quills in the dogs: A review and case series. Can Vet J 36:488-893, 1995
5. Pfleghaar S, Sch+�-�ffer EH: Die linseninduzierte Uveitis (Endophthalmitis phakoanaphylactica) beim Haustier. Tierd Prax 20:7-18, 1992
6. Spiess BM, R+�-+hli MB, Bollinger J. Augenverletzungen durch Katzenkrallen beim Hund. Schweiz Arch Tierheilk 138:429-433, 1996
7. Van der Woerdt A, Nasisse MP, Davidson MG: Lens-induced uveitis in dogs: 151 cases (1985-1990). J Amer Vet Med Assoc 201:923-926, 1992
8. Wilcock BP, Peiffer RL, Jr: The pathology of lens-induced uveitis. Vet Pathol 24:549-553, 1987
9. Wilcock BP: Eye and ear. In: Jubb, Kennedy and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol 1, pp.481-485. Elsevier Limited, Philadelphia, PA, 2007