WSC Conference 7, case 2
Signalment:
6-year-old, male neutered American pit bull terrier, canine (Canis familiaris)
History:
The patient had chronically pruritic, erythematous, and hyperplastic skin with numerous comedones. A prior history of intermittent superficial and deep pyoderma was reported.
Gross Pathology:
Lesions were predominantly on the ventral abdomen, inner thighs, and cranial hind limbs, but were also present over the dorsum and top of the head.
Laboratory Results:
Superficial and deep skin scraping was performed and was negative for ectoparasites.
Surface cytology was performed and was reported as pyogranulomatous inflammation with the presence of cocci
Aerobic culture and sensitivity of the skin surface was performed and Staphylococcus pseudintermedius sensitive to most antimicrobials was cultured.
Microscopic Description:
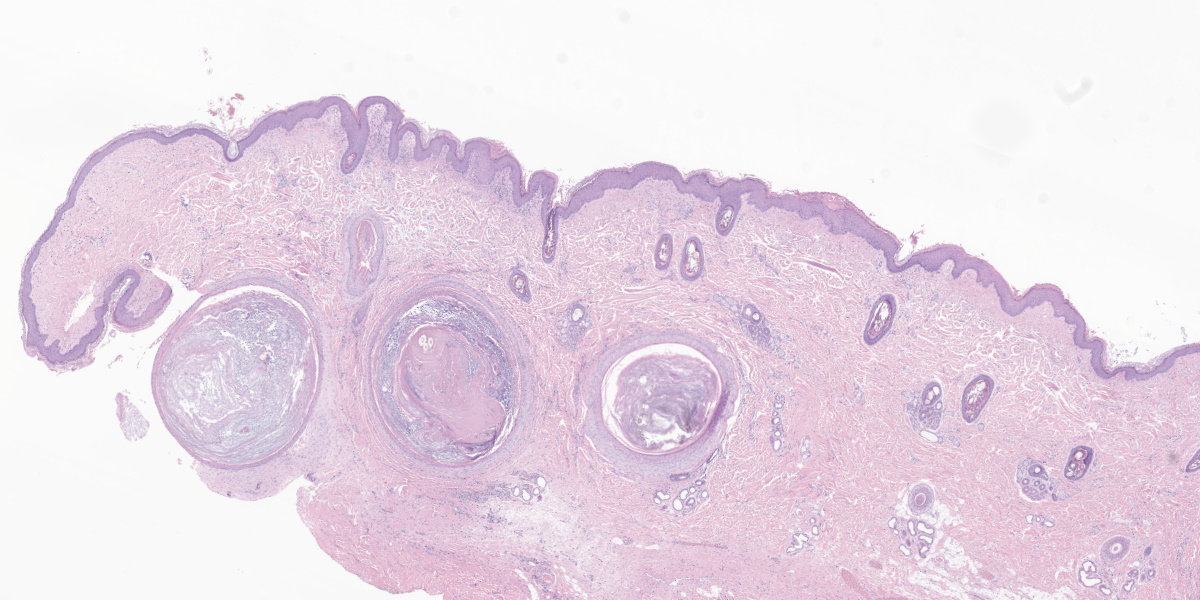
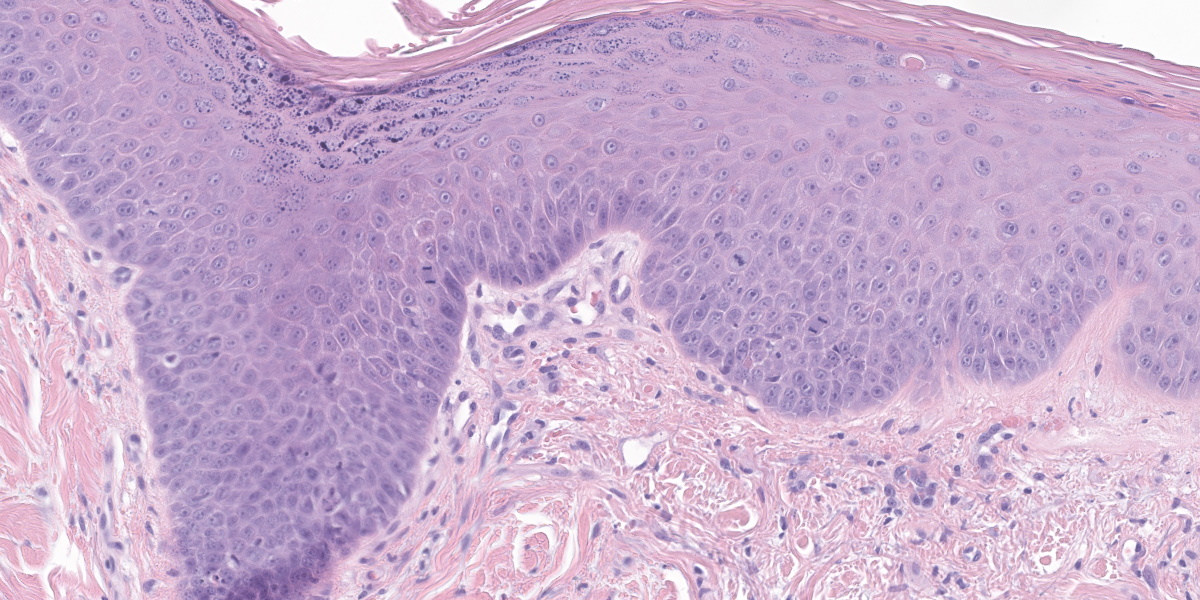
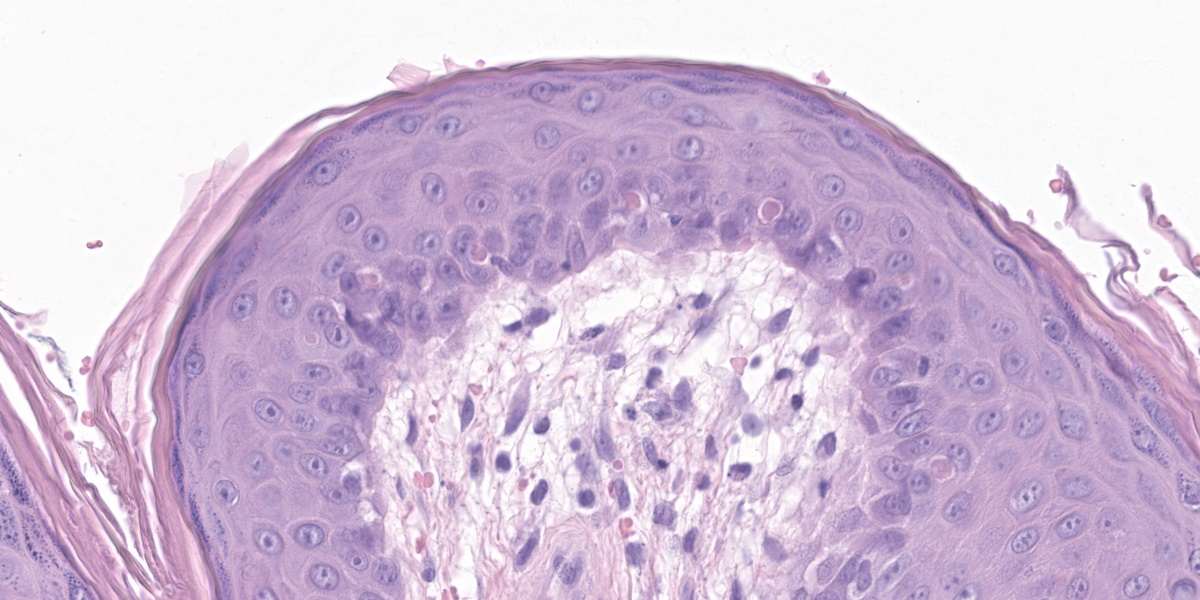
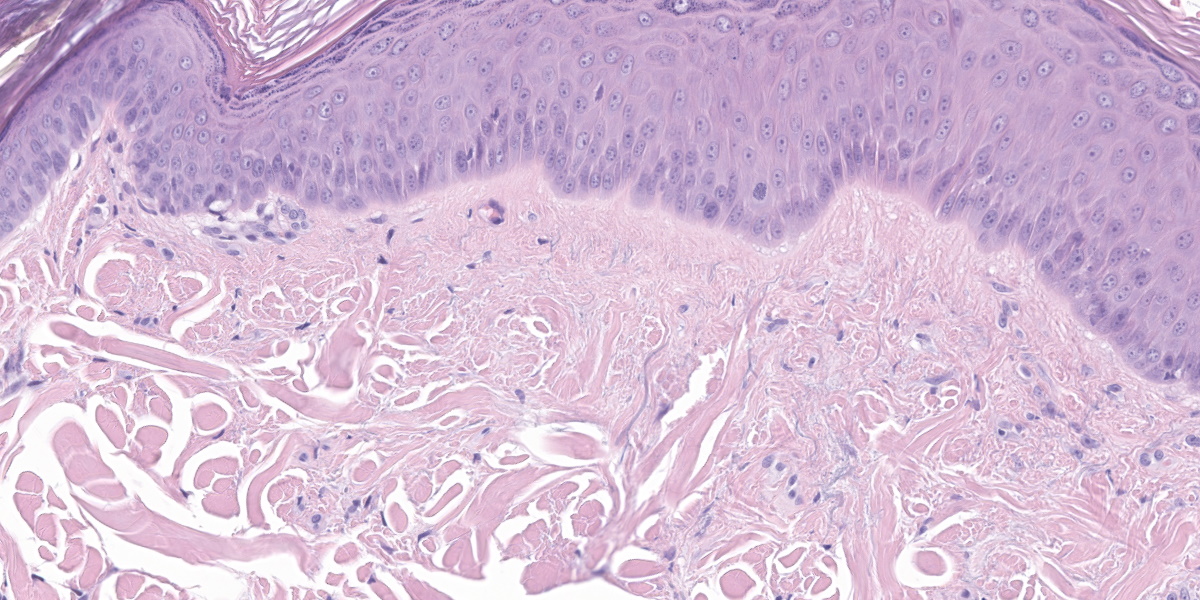
The epidermis is moderately hyperplastic with occasional dysplastic keratinocytes (increased nuclear to cytoplasmic ratio in the stratum spinosum) with suprabasal mitoses, and infrequent hypergranulosis. There are rare suprabasilar apoptotic keratinocytes (sunburn cells). There is multifocal parakeratotic hyperkeratosis. Follicular infundibula are markedly expanded by keratin (comedones) and surrounded by a rim of fibrosis. The superficial dermis has numerous wavy basophilic fibers (solar elastosis) and superficial fibrosis. There is a mild perivascular and perifollicular infiltrate of lymphocytes, plasma cells, and histiocytes with multifocal edema. There is a small focus of free keratin surrounded by macrophages adjacent to one of the comedones. The other sample (not submitted) demonstrated comedone rupture with pyogranulomatous dermatitis.
Contributor’s Morphologic Diagnosis:
Haired skin: Epidermal dysplasia with solar elastosis, comedones, and mild dermatitis (actinic dermatosis).
Contributor’s Comment:
This case exhibits classic features of actinic dermatosis, also called solar dermatosis or actinic keratosis. Exposure to ultraviolet radiation (most often sunlight) leads to epidermal hyperplasia and dysplasia with scattered suprabasilar mitoses and apoptotic keratinocytes (“sunburn cells”). The stratum corneum often exhibits parakeratotic hyperkeratosis.
In the dermis, elastin fibers are degenerate and appear as thick, wavy, basophilic strands (solar elastosis). Laminar fibrosis occurs in the superficial dermis and extends around follicular infundibula, which dilate to form comedones. There may also be a layer of pale, smudgy collagen fibers in the superficial dermis. There is often a superficial perivascular infiltrate of lymphocytes, plasma cells, and fewer macrophages, neutrophils, and occasionally eosinophils. Comedones may rupture, leading to furunculosis, and lesions can also become secondarily infected. Some cases may exhibit vasculopathy or proliferation of superficial dermal vessels.2 The epidermis may exhibit hyperpigmentation, though this change is not observed in this patient’s nonpigmented skin.4
Because exposure to sunlight is the initial step in the pathogenesis of actinic dermatosis,
affected dogs are often known sunbathers and may live at low latitudes or high altitudes. Lesions are most common on sparsely haired areas, such as the ventral abdomen, inguinum, axilla, dorsal muzzle, and periorbital regions. Non-pigmented areas are also more commonly affected. Predisposed dog breeds include short-haired dogs such as pit bulls, boxers, whippets, Dalmatians, greyhounds and Italian greyhounds, beagles, and basset hounds.2
Actinic dermatosis is also commonly observed in cats, horses, cattle, and humans. In cats, predisposed locations include the face and pinnae. In horses and cattle, lesions are often found on the eyelids (including third eyelid), conjunctiva, and perineum.7 Grossly, actinic dermatosis appears as patchy erythema, crusts, macules, and papules, progressing to plaques and nodules that may be ulcerated. Comedones may be grossly visible as dark foci or small nodules. Multiple lesions are often present, as solar exposure occurs over a large area of the patient. 2
Several of the changes described above are protective mechanisms of the epidermis against damage caused by UV radiation. Melanin blocks UV radiation and absorbs free radicals.1,4 Hyperpigmentation of the epidermis occurs via an immediate redistribution of existing melanin or more chronically via increased melanin synthesis by melanocytes and increased transfer of melanin to keratinocytes, which appears histologically as “hats” over the keratinocyte nuclei.4 Epidermal hyperplasia and hyperkeratosis, stimulated by epidermal growth factors released after UV exposure, provides an increased physical barrier. 1
If protective mechanisms fail, the epidermal dysplasia of actinic dermatosis can progress to in situ and then frank squamous cell carcinoma (SCC). Dogs with solar-induced SCC may have a longer median survival time than dogs with SCC without evidence of solar damage.8 UV light causes cross-linking of pyrimidine nucleosides into pyrimidine dimers, which distort the structure of the DNA double helix, leading to DNA replication failure and cell death or transformation. 4 Additionally, investigation into the molecular basis for actinic dermatosis and carcinogenesis in humans demonstrates similar genetic dysregulation between actinic dermatosis and SCC and implicates UV-induced mutations in p53.5,6 Other solar radiation-induced neoplasms, such as hemangioma/hemangiosarcoma, may occur concurrently in animals with solar exposure.2,4 Basal cell carcinomas and squamous cell carcinomas in humans are both linked to UV exposure, and p53 mutations are commonly found in these tumors.3
Contributing Institution:
University of Pennsylvania
School of Veterinary Medicine
https://www.vet.upenn.edu/veterinary-hospitals/ryan-veterinary-hospital/services/diagnostic-laboratories
JPC Diagnosis:
Haired skin: Epidermal dysplasia, mild to moderate, with keratinocyte apoptosis, solar elastosis, and comedones.
JPC Comment:
As the contributor notes, DNA damage due to sunlight exposure is the inciting cause of actinic dermatosis and the malignant transformation of keratinocytes that may occur with prolonged exposure. Sunlight contains three types of ultraviolet radiation that reach the earth’s surface: long wavelength UVA radiation in the 400-315 nm spectrum, UVB radiation (315 to 280 nm), and short-wave UVC radiation (280 to 100 nm).9 The vast majority of UV radiation that reaches the skin of humans and animals is UVA radiation, as all but approximately 5% of UVB radiation, and all UVC radiation, is absorbed by atmospheric ozone.1,9 Once UV light reaches the skin, host factors such as amount and type of hair and melanin pigmentation within the skin, as well as environmental factors, ultimately determine the amount of UV exposure received by the skin of a particular animal.9
UV radiation is considered a “complete carcinogen” in that it is both a mutagen, due to its direct effects on DNA, and a non-specific damaging agent, giving it properties of both a tumor initiator and a tumor promoter.1 In the skin, UVA penetrates deeply into the dermis and is efficient at generating reactive oxygen species that damage DNA via indirect photosensitizing reactions.1 In contrast, UVB is almost completely absorbed by the epidermis, where it is directly absorbed by DNA within keratinocytes, leading to molecular rearrangements, such as covalent bonds between adjacent thymine or cytosine bases (“pyrimidine dimers”).1,9 Most pyrimidine dimers are removed by DNA repair mechanism; however, some pyrimidine dimers escape this process and cause cells either to undergo apoptosis, leading to the “sunburn cells” seen in actinic keratosis, or to accumulate DNA mutations during subsequent replication, leading to neoplasia.1,9
The lesions of actinic dermatosis described by the contributor and well-illustrated in this case, are a result of chronic exposure to UVB and UVA radiation. At the outset, lesions appear grossly as areas of erythema with scaling and crusting, followed by the appearance of papular or plaque-like foci of thick, lichenified, erythematous crusted patches and plaques.4,9 Progression to in situ carcinoma, squamous cell carcinoma, or basal cell tumors may occur in areas of preneoplastic actinic dermatosis.9
Exposure to UV radiation can also damage skin through photosensitization. Photosensitization is skin damage due to the interaction of UV radiation and photodynamic chemicals within the skin.9 When UV radiation interacts with photodynamic chemicals, the released energy produces a range of reactive oxygen species that damage cell membranes, DNA, proteins, and organelles, causing cell activation, degeneration, and/or death.9
Photosensitization injury is classified into four types based on the origin of the photodynamic substance. In type I (primary) photosensitization, the photodynamic substance is ingested by the animal, usually in the form of plants or drugs, and then absorbed systemically and deposited into the skin. Type II photosensitization occurs via accumulation of endogenous hematoporphyrins due to the inability to properly metabolize heme pigments. Type III photosensitization occurs when phylloerythrin, a chlorophyll degradation product, accumulates in the skin; this is also known as hepatogenous photosensitization since deposition in the skin is due to the inability of the liver to disposing of phylloerythrin normally. Finally, type IV photosensitization refers to photosensitization for which the pathogenesis is unknown.4
A final way that UV radiation can damage skin is through photoallergy. Photoallergy is distinct from photosensitization and occurs when an exogenous photodynamic substance functions as an antigen.4,9 Gross and histologic pathology is typically due either to an immediate, Type I hypersensitivity reaction or a delayed, Type IV cell-mediated hypersensitivity reaction in which the photoactivated substance acts either as a hapten or an allergen.9
Conference participants appreciated the impressive comedones in the examined section. Actinic comedones such as these can be distinguished from regular comedones by their concentric, peripheral fibrosis and the lack of attenuation of the outer root sheath. Inflammation resulting from the rupture of an actinic comedone is sometimes called solar furunculosis.
The moderator also pointed out the substantial dysplastic changes evident within the epithelium, including suprabasilar mitotic activity, irregularly piling up of keratinocytes, and the formation and elongation of rete ridges. In human medicine, these changes might be enough for a diagnosis of in situ squamous cell carcinoma; however, veterinary medicine tends to be more cautious of making this leap. The moderator noted that the epithelium appeared to be well on its way to malignant transformation.
Finally, the moderator discussed the importance of differentiating feline actinic dermatosis from Bowenoid in situ carcinoma (BISC), which can exhibit more aggressive and invasive biological behavior. Compared to actinic dermatosis, in BISC, keratinocytes in all layers of the epidermis typically appear more basaloid, rete ridges tend to be more bulbous and extend farther into the dermis, and dysplastic changes extend to the follicular outer root sheath.
References:
- D'Orazio J, Jarrett S, Amaro-Ortiz A, et al. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222-48.
- Gross TL, Ihrke PJ, Walder EJ, Affolter VK, eds. Skin Diseases of the Dog and Cat: Clinical and Histopathologic Diagnosis. 2nd ed. Blackwell Science Ltd; 2005:136-160.
- Lacour JP. Carcinogenesis of basal cell carcinomas: genetics and molecular mechanisms. Br J Dermatol. 2002;146 Suppl 61:17-9.
- Mauldin EA and Peters-Kennedy J. Integumentary System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Elsevier; 2016:509-736.
- Nelson MA, Einspahr JG, Alberts DS, et al. Analysis of the p53 gene in human precancerous actinic keratosis lesions and squamous cell cancers. Cancer Lett. 1994: 85(1):23-9.
- Padilla RS, Sebastian S, Jiang Z, et al. Gene expression patterns of normal human skin, actinic keratosis, and squamous cell carcinoma: a spectrum of disease progression. Arch Dermatol. 2010:146 (3):288-93.
- Valli VE, Bienzle D, Meuten DJ, Linder KE. Epithelial and Melanocytic Tumors of the Skin. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. John Wiley & Sons;2017:88-141.
- Willcox JL, Marks SL, Ueda Y, et al. Clinical features and outcome of dermal squamous cell carcinoma in 193 dogs (1987-2017). Vet Comp Oncol. 2019:17 (2):130-138.
- Welle MM, Linder KE. The Integument. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th ed. Elsevier; 2022:1154-1156.