Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 22
5 April, 2019
CASE III: NP545M3 (JPC 4074226)
Signalment: Canine; Cairn terrier, 9y9m, male-castrated.
History: History of progressive lameness of the right front limb, treated with phenylbutazone and prednisone for several weeks. At presentation in a referral center, the dog showed a stiff gait, lameness of the right front limb and pain on palpation of the axillary region. Supraspinatus muscle biopsy submitted.
Gross Pathology: Subgross examination of the fixed muscle showed round blackish structures/organisms scattered throughout the surface.
Laboratory results: Clinical chemistry revealed increased CK values and Anaplasma titer of 1:2560.
Microscopic Description:
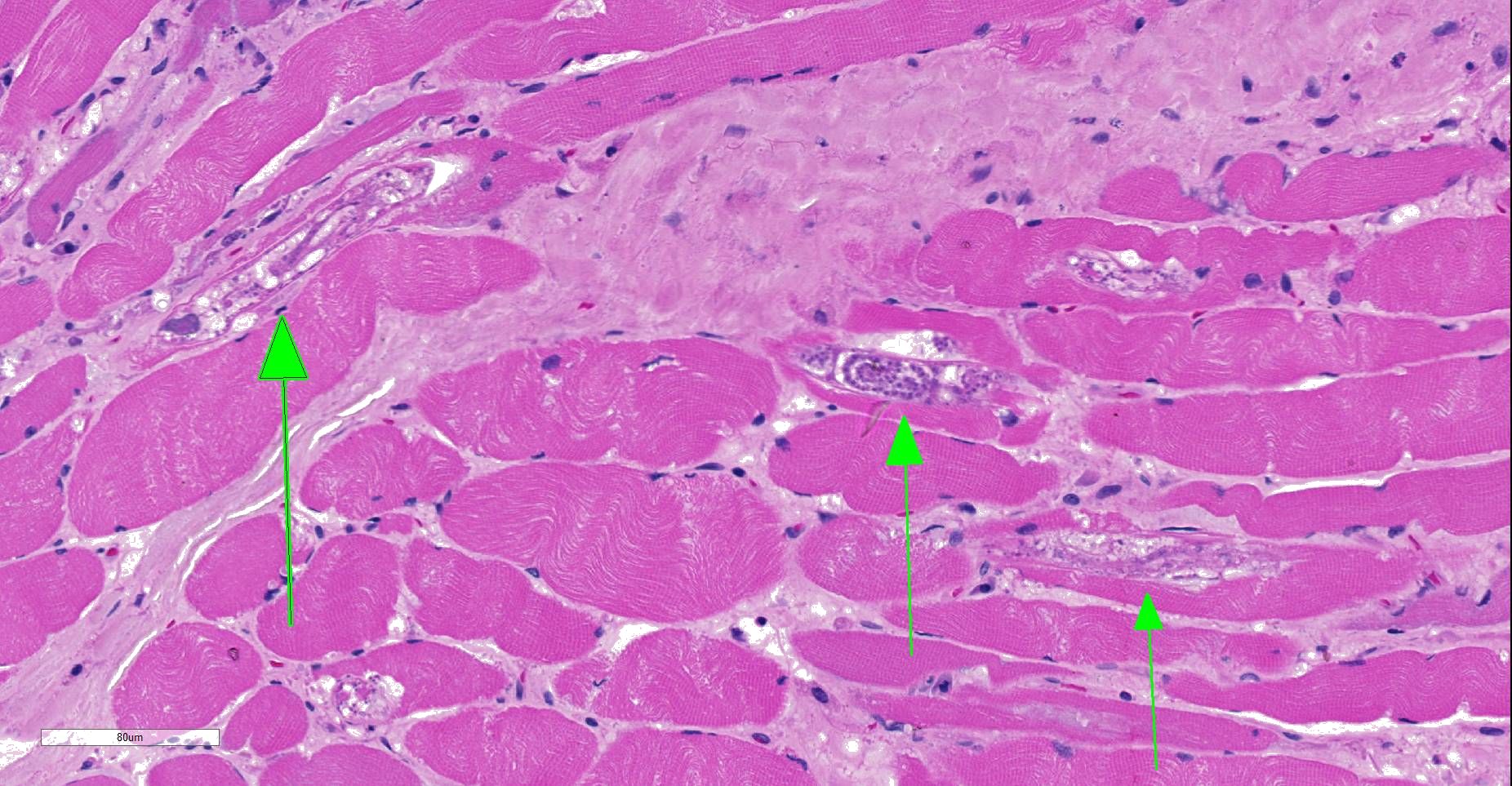
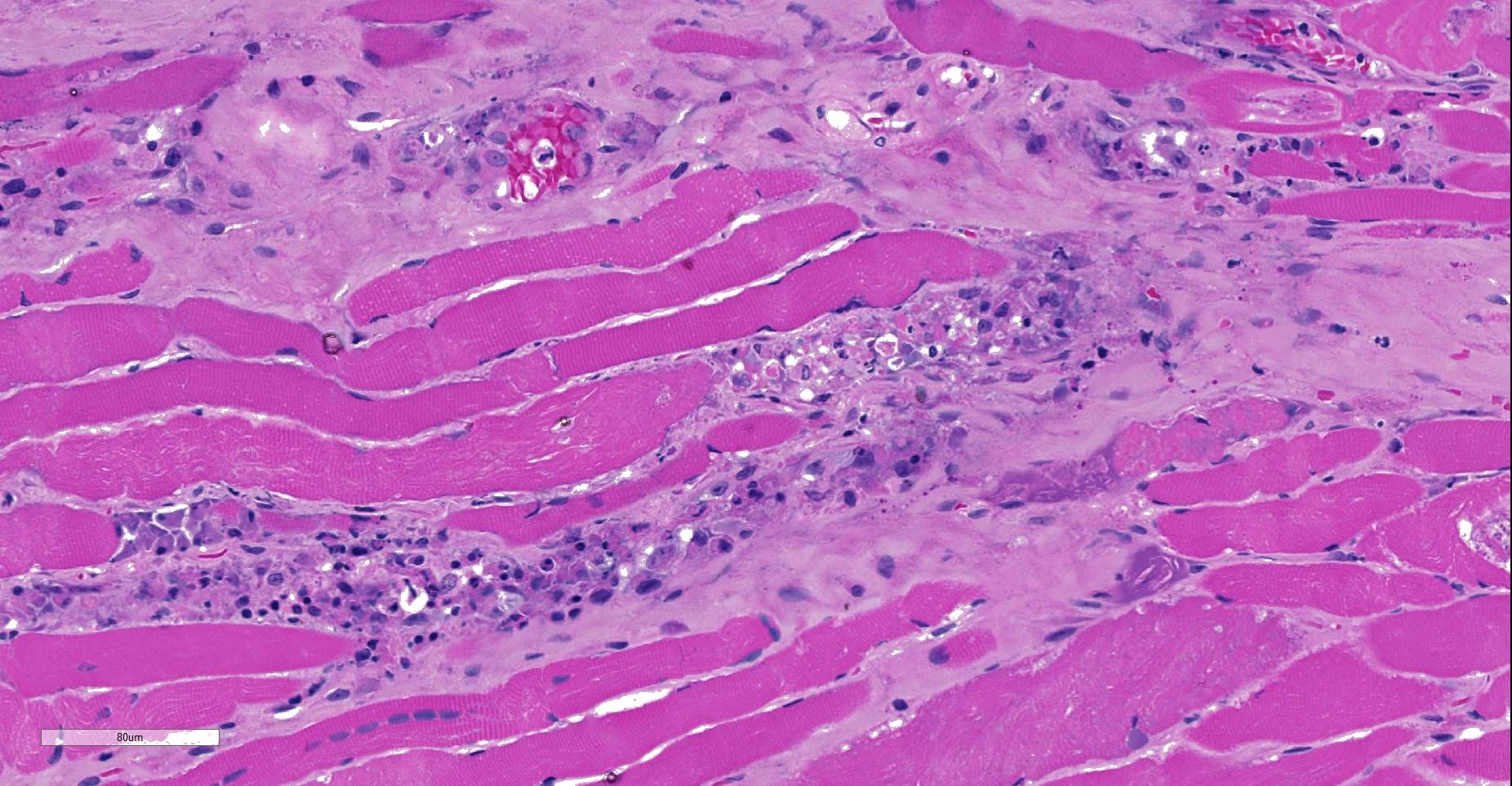
Moderately to poorly preserved muscle tissue showing moderate endomysial and perimysial fibrosis and mild multifocal muscle replacement by adipose tissue. Throughout the fascicles there are mild multifocal partially necrotizing mixed-cellular inflammatory infiltrates with predominance of histiocytes, macrophages and plasma cells accompanied by a few polymorphonuclear neutrophils. These mostly are seen in the interstitium and occasionally center on and invade necrotic myocytes. Throughout all fascicles there are numerous myocytes laden with 1-3 uncoiled sarcoplasmic nematode larvae of about 25-35 µm diameter, which in some sections show stichosomes.
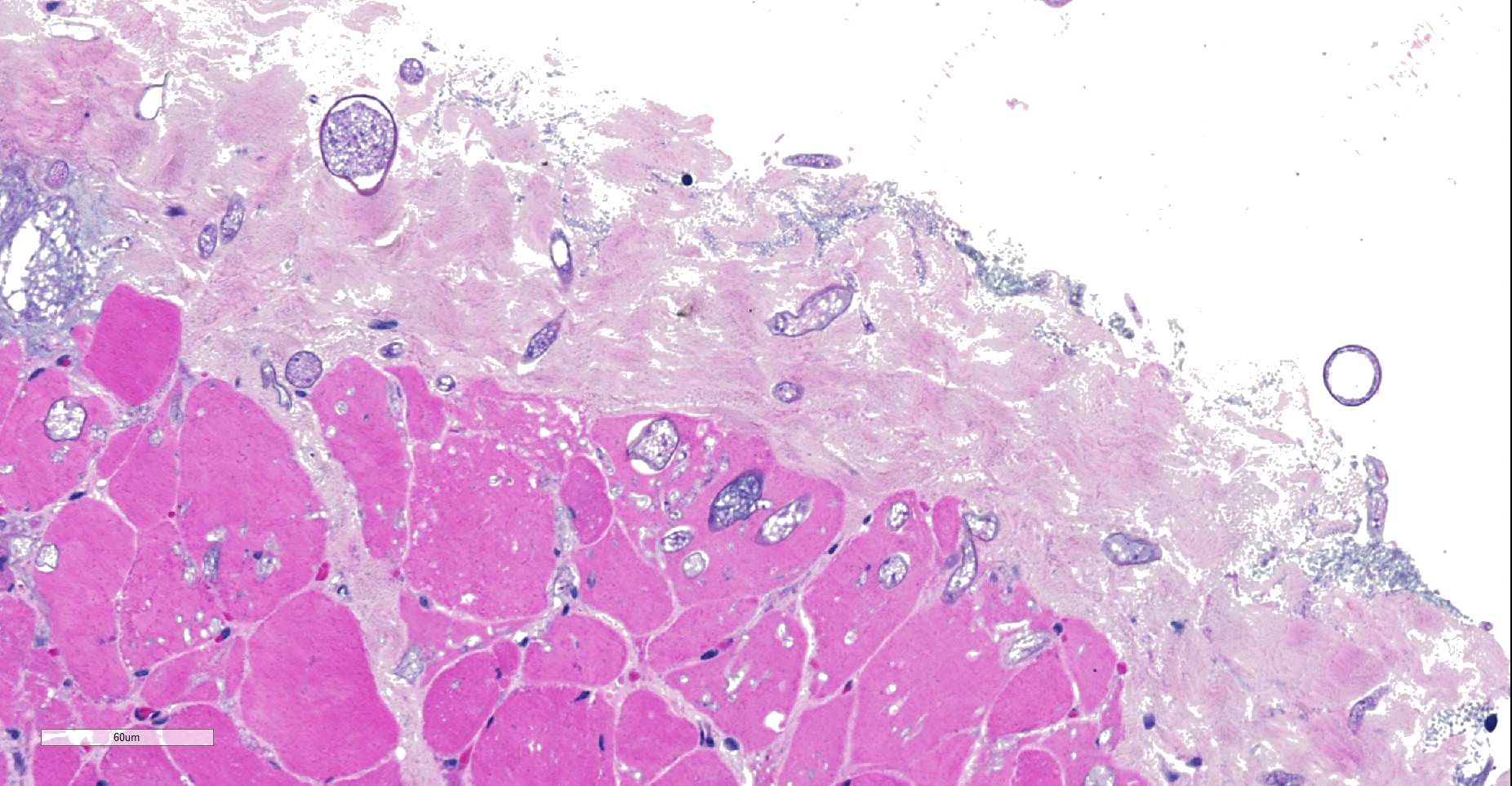
Throughout muscle fascia and most superficial aspects of myofascicles, there are mycotic organisms with numerous sporangia, sporangiophores and sporangiospores, as well as some columellae. The mycelium consists of mainly aseptate (coenocytic) hyphae with mostly non-parallel walls. In some slides, moreover, smaller, slender septated hyphae with dichotomous branching are seen. Fungal organisms, even in the depth of the tissue, are not accompanied by reactive tissue changes.
In addition to the fungal infestation, the surface of the biopsy shows massive bacterial colonization comprising rods, fusiform and coccoid bacteria.
Contributor’s Morphologic Diagnosis:
- Myositis, mixed-cellular, multifocal, subacute, moderate, associated with intramysial trichurid larvae (Trichinella spp.).
- Superficial myofascial mucormycosis, non-reactive, diffuse, acute.
- Bacterial contamination, mixed flora, superficial, severe.
Contributor’s Comment: Even though definite histological classification is not possible, the uncoiled character and absence of nurse cells very much indicates an infection by Trichinella pseudospiralis, the only other common species seen in tempered climate zones. Other metazoic invaders of the muscle (as hookworms) usually do not infest within the sarcoplasma. Retrospective analysis of the medical history revealed the dog to be severely immunocompromised, which explains both the minimal inflammatory tissue reaction and absence of any significant inflammation in areas invaded by the described fungi. As the sample was severely autolytic and contaminated by bacteria, the presence of fungi first was considered a contamination, as well. Consultancy sought at the Federal Center for Mycotic diseases, however, excluded the possibility that this massive infestation and the development of sporangia could have resulted from a contamination within less than 36 hours. Hence, the authors consider the infection to have taken place in vivo. This assumption appears to have gained support by successful treatment of the dog with antimycotics after withdrawal of corticosteroids. Based on morphology and later confirmed by sequencing of nuclear ribosomal internal transcribed spacer (ITS) region3 fungal organism were identified resembling Mucor spp. The infection may have been opportunistic as a consequence to immunosuppression.
Contributing Institution:
Institut of Veterinary Pathology LMU, Munich
Veterinaerstr. 13; 80539 Munich
Germany
www.patho.vetmed.uni-muenchen.de
JPC Diagnosis: Skeletal muscle: Degeneration and necrosis, multifocal, mild, with marked edema and intramyocyte nematode larvae, consistent with Ancylostoma caninum.
JPC Comment: Careful consideration was given to the contributor’s diagnosis of trichinosis in this case, however, we prefer a diagnosis of ancylostomiasis in this case. The larvae in this section are not encysted; Trichinella sp. often are surrounding by a hyaline cyst wall ranging to 10um during the formation of “nurse cells”. Within nurse cells, Trichinella sp. are generally coiled, while many of these larvae are present in longitudinal section. Larvae of Trichinella species also have a prominent stichosome and bacillary bands, which are absent in these larvae, which also possess lateral cords, vice the hypodermal bands seen in Trichinella sp. Several of the larvae also have a prominent flask-shaped esophagus characteristic of Ancylostoma. 1
Inatome et al. first identified that third-stage larvae of Ancylostoma caninum may migrate through muscle tissues and occasionally will take up residence in myofibers. Other researchers have identified other members of this genus, including A. braziliense and A. tubaeformae in host striated muscle. A detailed morphologic analysis of this finding was completed in 1975 by Lee and Beaver following oral inoculations of the mouse, cat, and monkey. In affected muscles, there are a number of degenerate and necrotic fibers, suggesting that the residence of an individual larva is not permanent within any given myofiber, and that larvae may move from one fiber to another, utilizing myofibers as a convenient way to evade the host’s immune response.
A number of other nematode larvae have also been identified within striated muscle, to include Trichinella which undergoes significant growth after penetration and the formation of a cyst around it to protect it from the immune response. Larva of Toxocara canis will also migrate through muscle tissue but does not infiltrate myofibers, instead forming fibrous cysts within connective tissues.
We agree with the contributor regarding the presence a zygomycete fungus at the edge of the section, which do not appear to be the subject of an immune response, suggesting that they may be contaminants in this case.
References:
- Gardiner CH, Poynton SL. In: An Atlas of Metazoan Parasites in Animal Tissues. Washington DC, American Registry of Pathology, 1999: 41-44.
- Lee, KT, Little MD, Beaver PC. Intracellular (muscle-fiber) habitat of Ancylostoma caninum in some mammalian hosts. J Parasitol 1975, 61(4): 589-598.
- Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Fungal Barcoding C, Fungal Barcoding Consortium Author L: Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci U S A 109: 6241-6246, 2012