Signalment:
Over the course of a 3-day hospitalization, the alpaca was noted to be anxious, depressed, and colicky with decreased gastrointestinal motility and intermittent recumbency (both sternal and lateral). He was treated intensively with fluids, antibiotics and supportive care. Facial and pulmonary edema, oliguria and azotemia developed over the 3 days with initial partial response to diuretics followed by apparent anuria. Sinus tachycardia was present initially, but by early on day 3 arrhythmias were noted. Ventricular tachycardia (up to 190 bpm) with runs of premature ventricular contractions developed and worsened in spite of I.V. lidocaine. The alpaca went into cardiac arrest and could not be resuscitated.
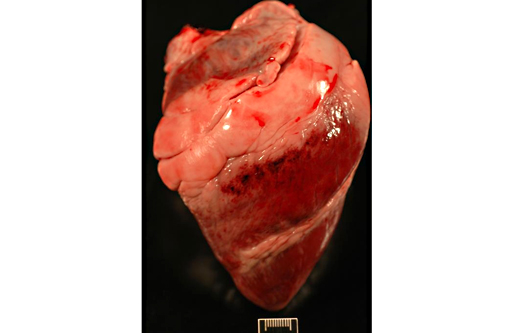
Gross Description:
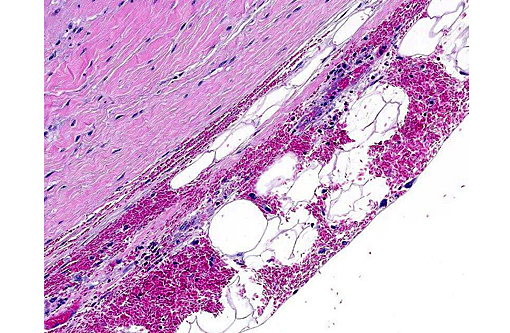
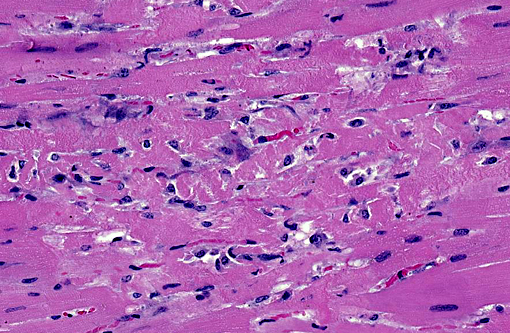
Histopathologic Description:
Heart (left ventricular myocardium): Multiple foci of hemorrhage are present in the subendocardial and subepicardial myocardium. Myofibers in these foci and in scattered foci throughout the sections are pale, granular to fibrillar or fragmented and the endomysium and perimysium are expanded by a combination of edema and cells with variably pyknotic to plump nuclei. Adjacent myofibers occasionally have centrally located hypertrophied nuclei. The endocardial hemorrhage in two sections surrounds Purkinje fibers which are vacuolated and fragmented. Scattered individual myofibers are hyalinized and are brightly eosinophilic (Zenkers necrosis) or lightly basophilic (early mineralization). Rarely, necrotic fibers are surrounded by neutrophils or mononuclear cells (myophagia).
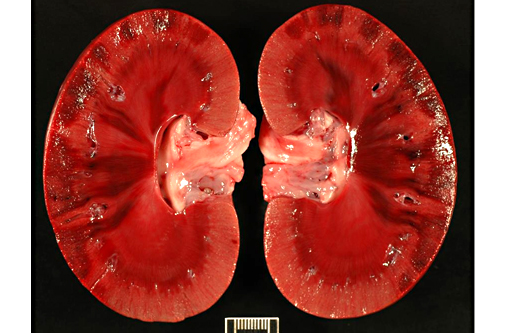
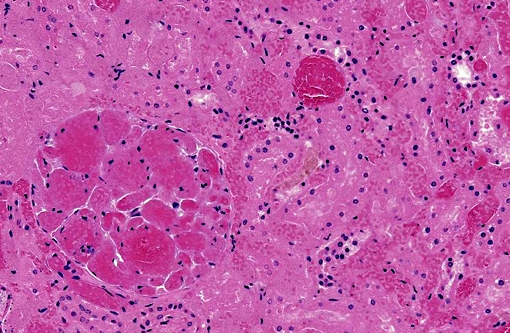
Kidney: There are large wedge shaped areas of acute infarction characterized by hemorrhage and coagulative necrosis of the cortex and medulla. Cells of the infracted glomeruli and tubules have loss of cellular detail and karyolytic nuclei. Glomeruli multifocally contain abundant fibrin within capillaries. Small numbers of renal tubules exhibit acute tubular necrosis in which epithelial cells are hypereosinophilic, have pyknotic nuclei, and slough into the lumen (cellular casts). Surrounding tubular epithelium is multifocally attenuated. There is multifocal mineralization of necrotic tubules.
Morphologic Diagnosis:
1. Heart (left ventricle): Moderate multifocal subacute myocardial degeneration and necrosis with associated hemorrhage compatible with oleandrin intoxication.
2. Kidneys: Severe multifocal acute infarction and multifocal acute tubular necrosis.
Additional Diagnoses (Tissue Not On Slide):
- Stomach (C3), small intestine and colon: Moderate diffuse congestion and multifocal transmural hemorrhage with multifocal superficial necrosis.
- Adrenals: Severe multifocal subacute medullary hemorrhage and necrosis.
- Pelvic limbs: Mild multifocal myonecrosis with hemorrhage and multifocal myofiber regeneration.
Lab Results:
Condition:
Contributor Comment:
The cardioactive glycosides of oleander exert a positive inotropic effect, caused by interference with normal calcium channel mechanisms and eventual intracellular accumulation of calcium.(10) The underlying mechanism for renal failure is undetermined at this time, but is a common finding with oleander toxicity in New World camelids.(6) Two possible mechanisms include inhibition of the Na+-K+ ATPase pump in the renal tubules causing direct tubular injury, or infarction due to hypoperfusion secondary to cardiac damage. It is also possible that it is a combination of both mechanisms, as in this case both infarction and acute tubular necrosis were observed.
Other cardiotoxic plants, zoo or wild animals that might be exposed include: foxglove (Digitalis lannata or D. purpurea), yellow oleander (Thevetia peruviana), rhododendrons and azaleas (Rhododendron spp.), mountain laurel (Kalmia latifolia), kalanchoe (Kalanchoe blossfeldiana and its hybrids), milkweeds (Asclepias species), lily of the valley (Convallaria majalis), Dogbane (Apocynum cannabinum), avocado (Persea Americana Guatamalan variety). Other cardiotoxins include ionophores such as monensin and salinomyocin, which was responsible for a large alpaca mortality event through contamination of a commercial feed.(5)
JPC Diagnosis:
1. Heart, myocardium: Necrosis and degeneration, multifocal, moderate.
2. Heart, epicardium: Hemorrhage, acute, multifocal.
3. Kidney, cortex: Infarcts, acute, multifocal and coalescing.
Conference Comment:
To further complicate the picture, ischemic myocardial necrosis also occurs in disseminated intravascular coagulopathy, vitamin E/selenium deficiency, and inflammatory diseases such as periarteritis nodosa. Subendocardial necrosis is observed following acute brain injury (brainheart syndrome) or in catecholamine excess from a functional pheochromocytoma.(9) The presence of renal necrosis does not assist in narrowing down the list, as the potential candidates for inducing renal infarcts or toxic tubular necrosis is equally extensive with significant overlap from the cardiotoxic differentials. As the contributor points out, identifying the geographic location as California in the present case significantly elevates oleander from others on the list. The gross and microscopic findings of endocardial hemorrhage, while considered non-specific at best, have been reported as commonly occurring in oleandrin toxicity(4), is prominent in the distributed sections, and may have yielded some assistance in narrowing this relatively long list of ruleouts.
Some discussion among participants revolved around the presence of apparent vascular necrosis of some larger arteries and whether to attribute those to vasculitis, injury due to hypertension or the renal infarct, with the most favorable opinion being secondary injury from a period of hypertension.
Oleandrin toxicity is most often associated with horses, though it is known to be toxic to many species. Participants briefly contrasted the manifestations of toxin ingestion as reported in horses with the current case. In the horse, mortality appears to be more common, with a rate of 50% (30 cases) versus 25% in camelids (12 cases). The clinical signs between species are similar, with the triad of gastrointestinal, cardiac and renal disease consistently present in each species.(7,12)
References:
1. Ada SE, Al-Yahya MA, Farhan AH. Acute toxicity of various oral doses of dried Nerium oleander leaves in sheep. Am J Chin Med. 2001;29:525532.
2. Aslani MR, Movassaghi AR, Mohri M, Abbasian A, Zarehpour M. Clinical and pathological aspects of experimental oleander (Nerium oleander) toxicosis in sheep. Vet Res Commun. 2004;28(7):609-16.
3. Barbosa RR, Fontenele-Neto JD, Soto-Blanco B. Toxicity in goats caused by oleander (Nerium oleander). Res Vet Sci. 2008;85(2):279-81, 26.
4. Galey FD, Holstege DM, Plumlee KH, Tor E, Johnson B, Anderson ML, Blanchard PC, Brown F. Diagnosis of oleander poisoning in livestock. J Vet Diagn Invest. 1996;8:358-364.
5. Hughes KJ, Dart AJ, Hodgson DR. Suspected Nerium oleander (oleander) poisoning in a horse. Aust Vet J. 2002;80:412415.
6. Kosal DE, Anderson ME. An unaddressed issue of agricultural bioterrorism: A case study on feed security. J Anim Sci. 2004;82:33943400.
7. Kozikowski TA, Madgesian KG, Puschner B. Oleander intoxication in New World camelids: 12 cases (1995-2006). JAVMA. 2009;235:305-309.
8. Langford SD, Boor PJ. Oleander toxicity: An examination of human and animal toxic exposures. Toxicology. 1996;109(1):1-13.
9. Maxie MF, Robinson WF. Cardiovascular system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Philadelphia, PA: Elsevier Saunders; 2007:33.
10. Omidi A, Razavizadeh AT, Movassaghi AR, Aslani MR. Experimental oleander (Nerium oleander) intoxication in broiler chickens (Gallus gallus). Hum Exp Toxicol. 2011 May 16. [Epub ahead of print]
11. Poindexter BJ, Feng W, Dasgupta A, Bick RJ. Oleandrin produces changes in intracellular calcium levels in isolated cardiomyocytes: A real-time fluorescence imaging study comparing adult to neonatal cardiomyocytes. J Toxicol Environ Health A. 2007;70(6):568-74.
12. Renier AC, Kass PH, Magdesian KG, Madigan JE, Aleman M, Pusterla N. Oleander toxicosis in equids: 30 cases (1995-2010). JAVMA. 2013;242(4):540-549.
13. Schwartz WL, Bay WW, Dollahite JW, Storts RW, Russell LH. Toxicity of Nerium oleander in the monkey (Cebus apella). Vet Pathol. 1974;11(3):259-77.
14. Sharma P, Choudhary AS, Parashar P, Sharma MC, Dobhal MP. Chemical constituents of plants from the genus Nerium. Chem Biodivers. 2010;7(5):1198-207.
15. Soto-Blanco B, Fontenele-Neto JD, Silva DM, Reis PF, N³brega JE. Acute cattle intoxication from Nerium oleander pods. Trop Anim Health Prod. 2006;38(6):451-454.