Signalment:
Gross Description:
Electron Microscopic Description:
11-0193-1:
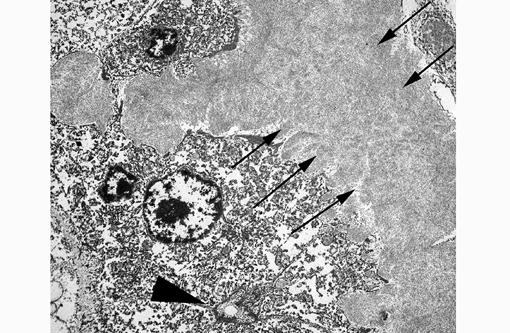
This electron micrograph contains portions of 5 large polygonal cells, 4 of which converge to form a 2 μm diameter channel lined by a microvillous brush border (bile canaliculus). The cell in the center of the image is 22 μm in greatest dimension and contains abundant, medium-electron dense, bland cytoplasm, and 2 round nuclei with prominent nucleoli and peripherally condensed heterochromatin (binucleate hepatocyte). The cells are bordered by a 15 μm wide branching sinusoid which is filled with large numbers of densely packed, pale, randomly-oriented and interlacing, non-branching, ~10nm diameter fibrils (amyloid).Â
11-0193-2:
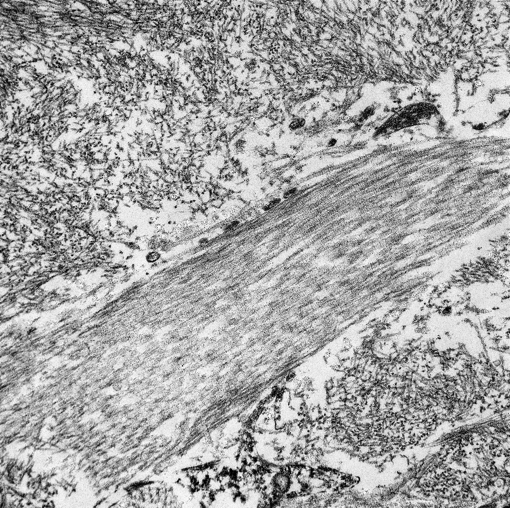
This electron micrograph contains 2 distinct types of extracellular fibrillar material. Centrally there is a 3 μm wide bundle of ~50nm diameter, banded fibrils arranged in regular parallel arrays (collagen). Above and below the collagen bundle, there are large numbers of densely packed, medium-electron dense, randomly-oriented and interlacing, non-branching, ~10nm diameter fibrils (amyloid). A small portion of a cell is visible at the bottom of the image.Â
Morphologic Diagnosis:
Condition:
Contributor Comment:
Amyloidosis is the pathologic accumulation of amyloid in tissues and can be systemic or localized. It has been identified in mammals, reptiles, and birds and is a well-recognized condition in rhesus monkeys and other macaques.(1,7,8,17)
By light microscopy, amyloid has a homogenous eosinophilic hyaline appearance which can be similar to other extracellular fibrillar proteinaceous deposits, such as collagen or fibrin. Ultrastructurally, however, it has a distinct appearance and can be readily differentiated, as seen in this case. When viewed with transmission electron microscopy, amyloid fibrils are non-branching, 7.5-10nm in diameter, and randomly-oriented and interlacing.(3) This is in contrast to collagen which has larger fibrils (~50nm in diameter) that are banded and arranged in regular parallel arrays; and fibrin, the strands of which vary in diameter and form dense irregularly-branching mats, generally in the vicinity of platelets.(4,5)
JPC Diagnosis:
Conference Comment:
Systemic AA amyloidosis can also be inherited in humans (familial Mediterranean fever), Chinese Shar-Pei dogs (autosomal recessive Familial AA amyloidosis), Abyssinians (autosomal dominant with incomplete penetrance) and Siamese cats. In Shar-Peis with inherited amyloidosis, amyloid is preferentially deposited in the renal medullary interstitium, while in Abyssinian cats, deposition is glomerular and in Siamese cats amyloid deposits accumulate in the hepatic space of Disse. Whether reactive or inherited, clinical signs in systemic amyloidosis are generally secondary to dysfunction of adjacent organs and pressure atrophy in areas adjacent to amyloid accumulation.(12,13)
In dogs, AA amyloidosis is usually idiopathic, but it has also been associated with Hepatozoon americanum, Ehrlichia can is and other causes of chronic suppurative/granulomatous inflammation. Canine AA amyloidosis lesions usually occur within renal glomeruli, which leads to proteinuria and progressive renal insufficiency.(13) In contrast, feline amyloidosis primarily affects the renal medulla, so proteinuria is not a typical clinical finding.(13) Equine systemic AA amyloidosis is a well-recognized hepatic lesion in horses used to produce hyperimmune serum.(13) Glomerular amyloidosis, with accompanying proteinuria, occurs occasionally in ruminants. Swine rarely develop amyloidosis.(13) Much like the other forms of reactive amyloidosis described above, avian amyloidosis tends to develop after a period of chronic inflammation and occurs most frequently in waterfowl, although it has also been reported in chickens (amyloid arthropathy).(9) Conversely, reactive amyloidosis is rarely reported in reptiles (snakes and tortoises).(17)
Reactive amyloidosis has been described in the Patas monkey, squirrel monkey, mandrill, chimpanzee, barbary ape and cynomolgus and rhesus macaques; incidence generally increases with age, likely due to cumulative exposure to parasites, infectious agents and injury.(7) Non-human primates with reactive amyloidosis often have concurrent arthritis and/or enterocolitis. Deposition typically occurs in the large or small intestine, or less commonly in the spleen, lymph node, liver, adrenal glands or stomach.(1,7) Affected animals often display elevated levels of alkaline phosphatase, aspartate aminotransferase, lactate dehydrogenase and serum amyloid A, in combination with decreased albumin and cholesterol on serum chemistry profiles.(8) Additionally, the cynomolgus macaque, rhesus macaque, squirrel monkey, gray mouse lemur and common marmoset have all been used as models for cerebral amyloidosis.(7)
Amyloidosis is a common age related change in laboratory mice, especially in CD-1 strains.(14) Mouse amyloidosis is typically composed of AA amyloid or AapoAII (senile amyloid) deposits, but mice are also subject to several localized forms of amyloidosis involving the brain or endocrine tumors. In contrast to AA amyloid, AapoAII deposition is less marked in the liver and spleen, with more deposition in adrenals, intestine, heart, lungs, thyroid and gonads. CBA and B6 mice are most susceptible to reactive (AA) amyloidosis, while A/J mice are resistant, and A/J and SJL mice are predisposed to AapoAII amyloidosis.(11) In hamsters, as in many other species, the incidence of amyloidosis increases with age. Interestingly, females are three times more likely to develop amyloidosis than males, likely due to a hamster female protein, which is similar to amyloid P. The liver, kidneys and adrenal glands are the most frequently involved organs.(11) Amyloidosis in gerbils has been experimentally associated with filariid infection, but it also occurs spontaneously in older animals. Similarly, renal amyloidosis is reported in aged rabbits, especially those that have been hyperimmunized for antibody production.(11)
As noted by the contributor, primary amyloidosis with AL amyloid is often associated with immune cell dyscrasias. Cutaneous amyloidosis occurs in approximately 10% of dogs and cats with extramedullary plasmactyomoas. Cutaneous amyloidosis in dogs is also associated with dermatomyositis and monoclonal gammopathy. In horses, the cause of nodular AL amyloid deposition within the skin and upper respiratory tract is unknown.(6)
In addition to AA, AL and Aβ amyloid discussed above, islet amyloid is significant in several veterinary species. Islet amyloid polypeptide (IAPP) produced by pancreatic islet β-cells in some species of mammals, birds and fishes. Although the exact pathogenesis is still unknown, IAPP can be deposited in pancreatic islets as amyloid in cats, non-human primates and humans, resulting in type 2 diabetes.(16) Endocrine disorders of the pancreas have been associated with amyloid deposits in the cynomolgus macaque,(10) rhesus macaque,(5) baboon, Celebes crested macaque, and Formosan rock macaque.(7)
References:
1. Blanchard JL, Baskin GB, Watson EA. Generalized amyloidosis in rhesus monkeys. Vet Pathol. 1986;23(4):425-430.
2. Cheville NF. Extracellular substances, pigments, and crystals. In: Cheville NF, ed. Ultrastructural Pathology: An Introduction to Interpretation. Ames, Iowa: Iowa State University Press; 1994:304-308.
3. Cheville NF. Extracellular Substances, Pigments, and Crystals. In: Cheville NF, ed. Ultrastructural Pathology: An Introduction to Interpretation. Ames, Iowa: Iowa State University Press; 1994:279.
4. Cheville NF. Blood and hemostasis. In: Cheville NF, ed. Ultrastructural Pathology: An Introduction to Interpretation. Ames, Iowa: Iowa State University Press; 1994:407.
5. De Koning EJ, Bodkin NL, Hansen BC, Clark A. Diabetes mellitus in Macaca mulatta monkeys is characterised by islet amyloidosis and reduction in beta-cell population. Diabetologia. 1993;36(5):378-384.
6. Ginn PE, Mansell JEKL, Rakich PM. Skin and appendages. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 1. Philadelphia, PA: Elsevier; 2007:662, 776.
7. Hukkanen RR, Liggitt HD, Anderson DM, et al. Detection of systemic amyloidosis in the pig-tailed macaque (Macaca nemestrima). Comp Med. 2006;56(2):119-27.
8. MacGuire JG, Christe KL, Yee JL, et al. Serologic evaluation of clinical and subclinical secondary hepatic amyloidosis in rhesus macaque (Macaca mulatta). Comp Med. 2009;59(2):168-73.Â
9. Murakami T, Inoshima Y, Sakamoto E, et al. AA amyloidosis in vaccinated growing chickens. J Comp Pathol. 2013;149(2-3);291-297.
10. OBrien TD, Wagner JD, Litwak KN, et al. Islet amyloid and islet amyloid polypeptide in cynomolgus macaques (Macaca fascicularis): An animal model of human non-insulin-dependent diabetes mellitus. Vet Pathol. 1996;33(5):479-485.
11. Percy DH, Barthold SW, eds. Pathology of Laboratory Rodents and Rabbits. 3rd ed. Ames, IA: Blackwell Publishing; 2007:93-94, 200-201, 301.
12. Snyder PW. Diseases of immunity. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, Missouri: Mosby Elsevier; 20012:37-38, 284-288.Â
13. Stalker MJ, Hayes MA. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals, 5th ed. Vol 2. Philadelphia, PA: Elsevier Saunders; 2007:315-316, 463-464, 532.
14. Thoolen B, Maronpot RR, Harada T, et al. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol. 2010;38(7 suppl):5S-81S.
15. Uno H, Alsum PB, Dong S, et al. Cerebral amyloid angiopathy and plaques, and visceral amyloidosis in aged macaques. Neurobiol Aging.1996;17(2):275-281.
16. Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91(3):795-826.
17. Zschiesche W, Jakob W. Pathology of animal amyloidoses. Pharmac Ther. 1989;41(1-2):49-83.