Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 23
15 April, 2020
CASE IV: S1601842 (JPC 4101199).
Signalment: Two week old, male Arabian foal (Equus caballus)
History: The foal was found in right lateral recumbency. It had appeared in good health the day before, although it was observed to fall once while nursing when the mare moved; however it immediately stood back up and resumed nursing. On physical examination the foal was afebrile, and had a heart rate of 100, and respiratory rate of 28. The foal was unable to rise, remain standing when assisted to rise, or maintain sternal recumbency. Front limb movement appeared hypermetric, the foal intermittently flailed with a rigid head and neck, and exhibited intermittent horizontal nystagmus. No external evidence of trauma was detected. The umbilical stump and limb joints appeared within normal limits, the abdomen appeared taut, and the sclerae were slightly yellow-tinged. Abdominal ultrasound revealed an intact urinary bladder, and possible thickening of the small intestinal wall.
The foal was euthanized the same day due to a poor prognosis.
Gross Pathology: The carcass was in good postmortem condition and well-fleshed. Conjunctival mucosa, sclera and gingival mucosa were slightly yellow tinged, and there was multifocal, moderate subcutaneous hemorrhage of the subcutis of right upper eyelid, and caudodorsal cranium (overlying interparietal and occipital bones). Cranial meninges were congested with two small, focal hemorrhages on the dorsolateral aspect of the right cerebral hemisphere.
The trachea contained scant stable foam, and the lungs were spongy and pink with mild, multifocal red mottling. There was a small dark red nodule of approximately 0.3cm in diameter on the cardiac right atrioventricular valve. The stomach contained a small amount of fragmented roughage (hay) and no milk curds. The cecum and large colon contained plentiful sand and the large intestinal mucosa was diffusely red. The small colon contained loose, malodorous feces.
Laboratory results: Rare mixed bacterial flora was isolated from liver on aerobic culture, and large numbers of Escherichia coli and mixed bacterial flora were isolated from small intestine and large colon. Salmonella PCR was negative on liver, small intestine and colon, and ELISA testing for both Clostridium perfringens toxins and Clostridium difficile toxins, was negative on small intestinal and cecal contents.
No parasite eggs were detected in feces. Heavy metal analysis of the liver detected a marginally deficient selenium concentration, while other tested minerals were within acceptable ranges for the species.
No aerobic bacterial pathogens were isolated from a submitted sample of dam?s milk, and Clostridium botulinum toxin testing was negative on a submitted feed sample (mare and foal pellet).
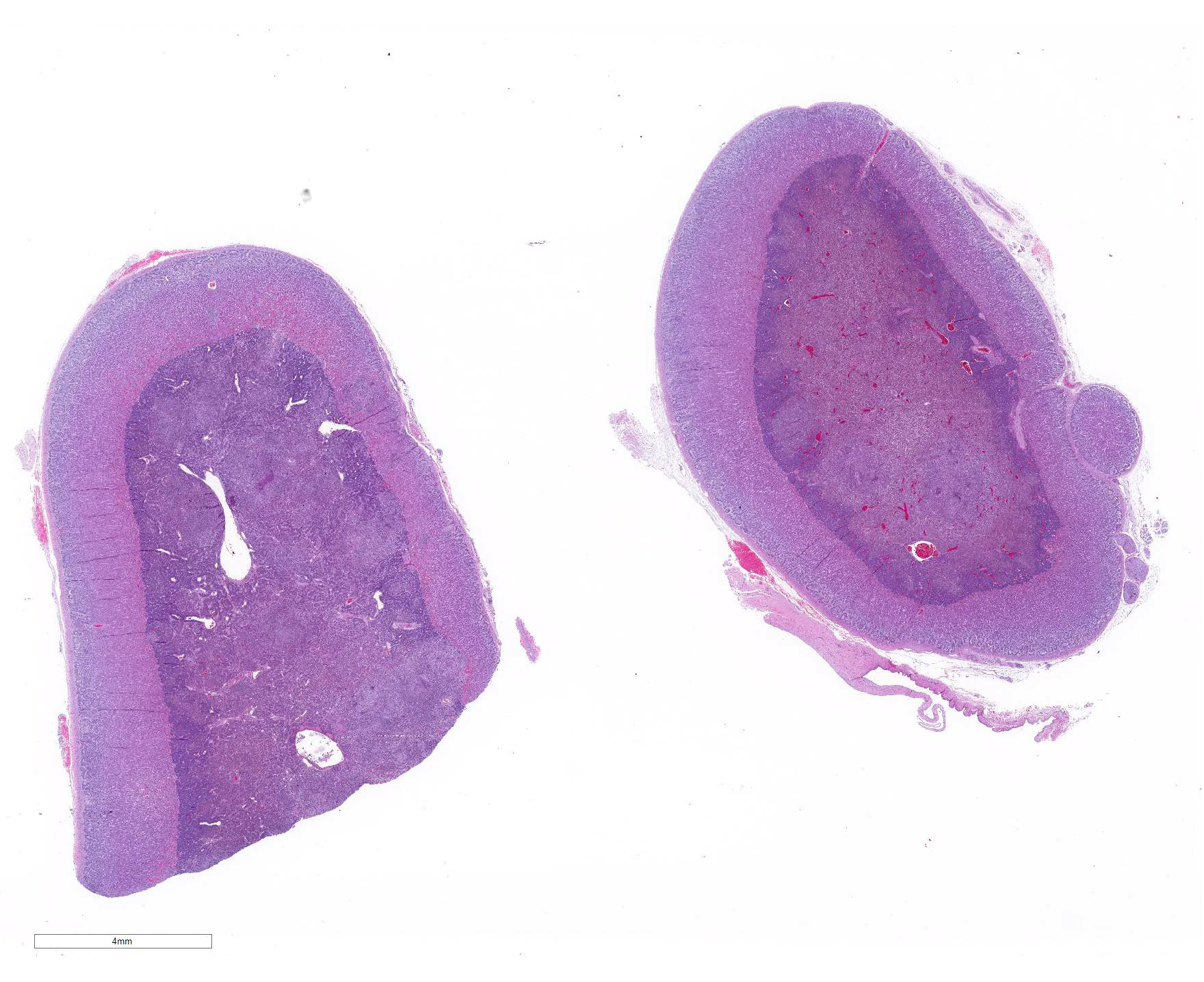
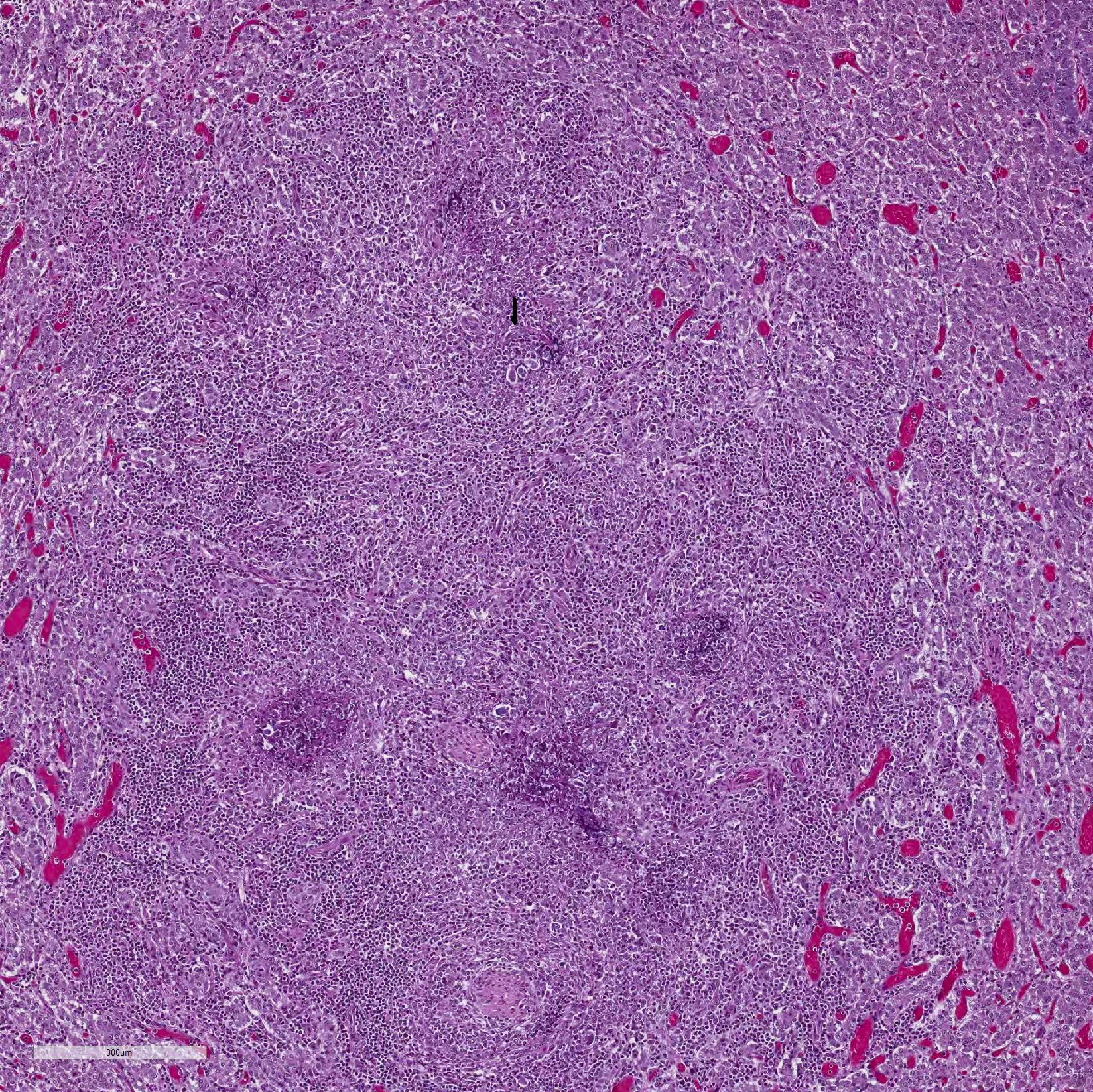
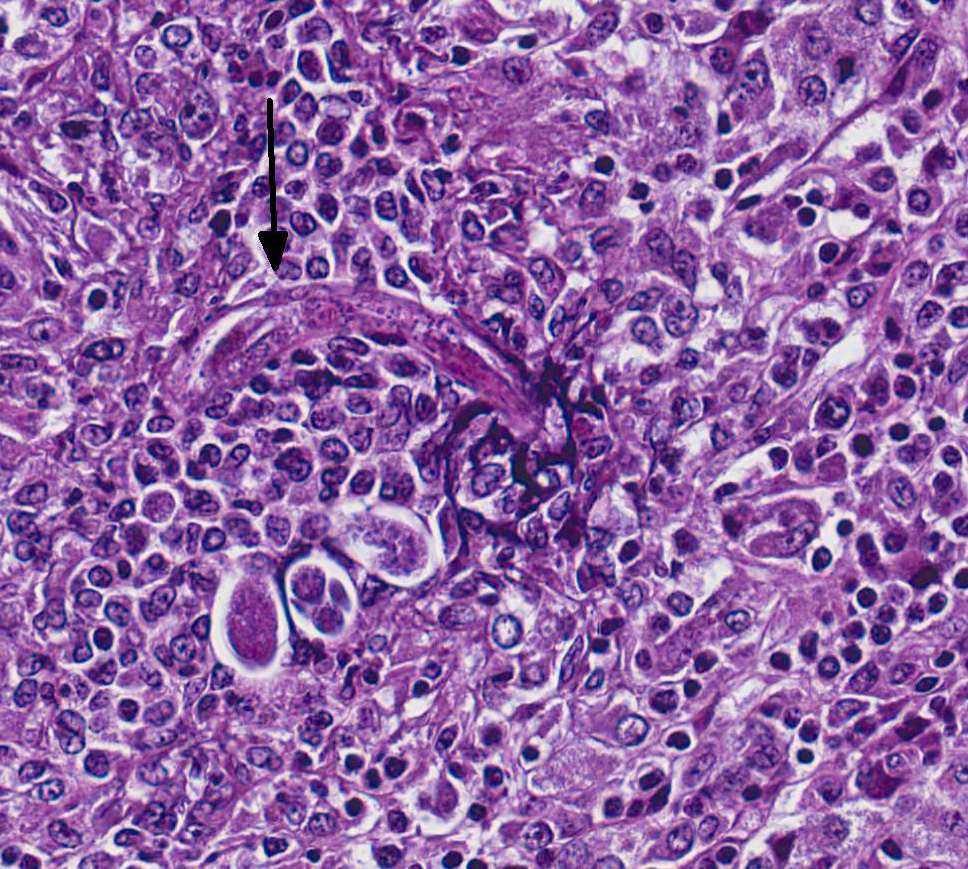
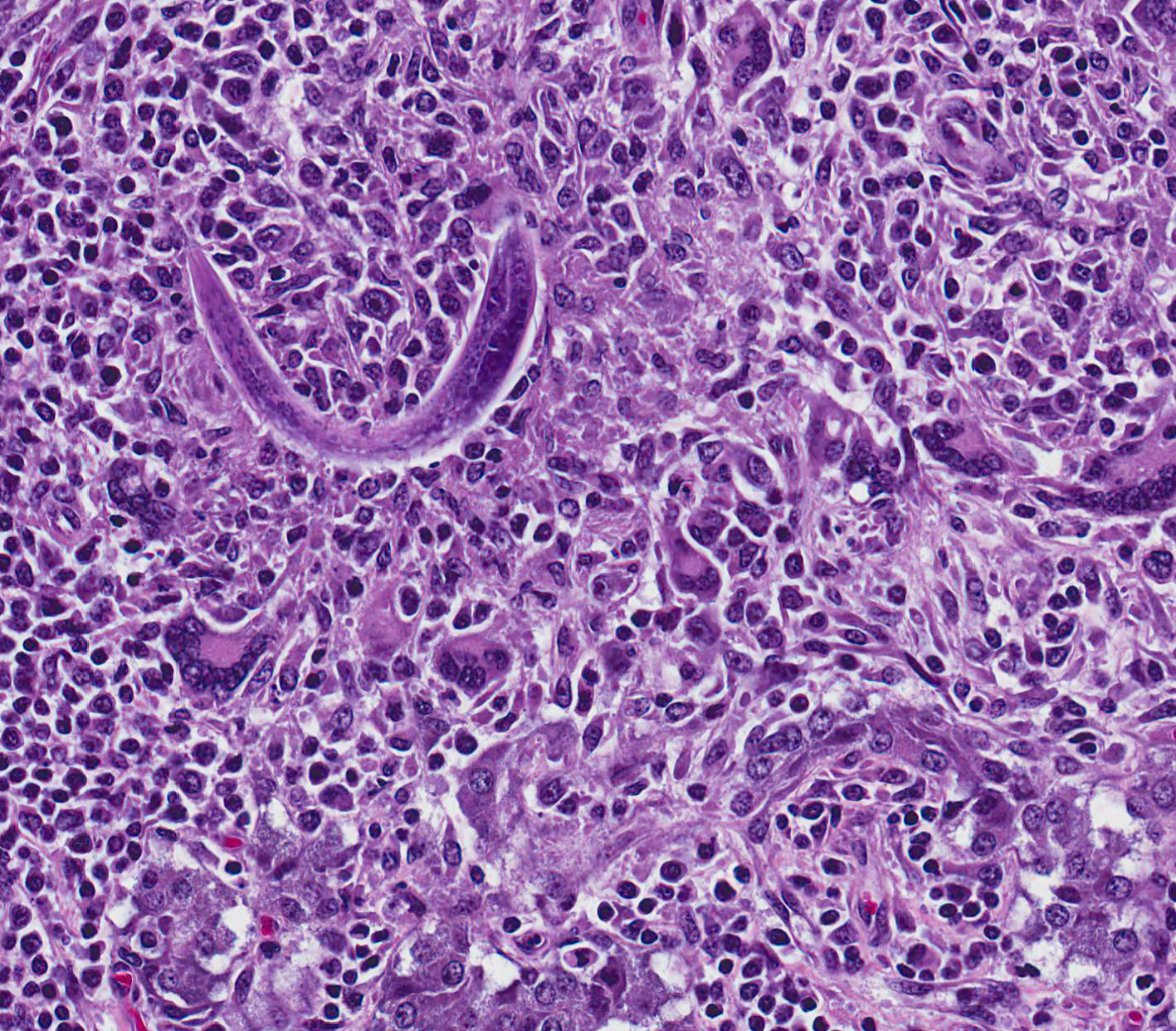
Microscopic Description: Adrenal gland: The medulla is extensively disrupted by multifocal to coalescent, variably sized foci of granulomatous inflammation characterized by densely cellular aggregates of Langhans-type multinucleated giant cells and epithelioid cells, and large numbers of admixed and/or peripheral lymphocytes and plasma cells. Some of the granulomas are centrally necrotic and there are scattered tangentially and cross-sectioned larval and adult rhabditid nematodes with a diameter of 10-25um, and fewer thin-walled, multicellular to larvated, ovoid eggs. In a small number of tangentially sectioned nematodes it is possible to identify anatomical features such as a rhabditiform esophagus with isthmus and terminal bulb, dark basophilic granular structures of 2-3 um within the pseudocoelom, and a smooth cuticle.
Contributor Morphologic Diagnosis:
Adrenalitis, granulomatous, lymphoplasmacytic, multifocal to coalescent, moderate to severe, with multifocal necrosis and rhabditiod nematodes, etiology presumptive Halicephalobus gingivalis.
Contributor Comment: In addition to the adrenalitis, histologic examination of the brain sections from this foal identified the presence of granulomatous to lymphohistiocytic meningoencephalitis, with multifocal malacia and rhaditid nematodes that were morphologically consistent with Halicephalobus gingivalis.
Halicephalobus gingivalis is a free-living nematode of soil, decaying organic matter and horse manure, and a facultative parasite primarily of horses and rarely of humans.5,7,9 although there is one published report of an outbreak of halicephalobiasis producing meningoencephalitis in a group of dairy calves in Denmark.3 Little is known about the life cycle and method of transmission; however it has been speculated that the portal of entry is mostly penetration of mucous membranes (particularly oropharyngeal following ingestion) and skin wounds, with subsequent, predominantly hematogenous spread to other organs.3,5,6,7,9 In one documented case of a fatal H.gingivalis infection in a 5 year old boy, he developed meningoencephalitis 8 days after falling into a horse manure spreader, acquiring manure-contaminated facial lacerations.7,9 Only female nematodes, larvae and eggs have been detected in tissues, and as such nematode reproduction in the host is thought to be asexual with parthenogenetic females. 3,5,6,7,9 Sexual reproduction is believed to occur during the free-living part of the life cycle, and both males and females have been found in the environment.3
In horses, the organs/tissues most commonly affected are the meninges/brain, with or without involvement of the spinal cord and central/spinal nerves, kidney, mandibular bone and maxillary bone/sinuses, gingiva, eye, and prepuce, with less frequent reports of involvement of adrenal gland, lung, mammary gland and testicle.5,7,9
Halicephalobus gingivalis infection of young foals is very rare.3,9 Strong evidence of a transmammary route of infection was documented in one case8 in which a 3-week-old foal presented with neurologic signs and was diagnosed postmortem with leptomeningoencephalitis caused by rhabditiod nematodes morphologically compatible with H.gingivalis. A biopsy of the dam?s mammary gland taken the previous year was diagnosed at the time as a nematode infection, and retrospectively as mastitis caused by infection with H.gingivalis.
Diagnosis of halicephalobiasis is mostly performed postmortem by histologic identification of consistent lesions and morphologically compatible rhabditoid nematodes; however the nematodes can also be extracted from macerated and sieved fresh tissue, washed, centrifuged and examined by light microscopy.3 If there is renal involvement, the nematode may also be shed in the urine, and can be collected by centrifugation of a urine sample. These methods enhance the ability to identify the characteristic features of adult female H.gingivalis including the rhabditiform esophagus with a corpus, isthmus and 2 esophageal bulbs, and the didelphic reproductive tract with reflexed ovary at the posterior end.7 Identification of the nematode can be confirmed by sequencing of extracted ribosomal DNA.3,6,7
Contributing Institution:
Disease Investigations
Institute for Conservation Research
San Diego Zoo Global
PO Box 120551
San Diego, CA 92112
http://institute.sandiegozoo.org/disease-investigations
JPC Diagnosis:
JPC Comment: The contributor has provided an excellent and comprehensive review of disease associated with Halicephalobus gingivalis. The genus Halicephalobus comprises eight species of small nematodes belonging to the Family Rhabditoida, which also includes Rhabdias, Strongyloides, and Pelodera.
The morphology of Halicephalobus is unique and
characteristic. Rhabditoids are one of three families which have platymyarian-coeloyarian
musculature, with the others being the strongyles and oxyurids.4 All
stages of the parasite possess the characteristic ?rhabditiform esophagus? ? a
unique structure with a corpus, isthmus and bulb. Halicephalobus adult
females possess a single genital tract as opposed to the paired tracts of Strongyloides.
For this reason, only one egg is seen per cross-section of an adult female.4
A fortuitous section can also demonstrate the dorsoflexed ovary mentioned by
the contributor, which is also unique to the genus.4
Only females of Strongyloides and Halicephalobus
are parasitic. Halicephalobus females (which are the only gender
recovered from lesions) are not hermaphroditic and likely not
parthenogenetic,? males live in decaying plant material, soil, and fresh and
salt water, as do both genders of the other seven species.2,5
Vectors have not been identified for this particular parasite; infections are
presumed to be the result of ingestion, inhalation, or direct contact with
infected plant matter or water with wounds or mucous membranes. Parasitic
females migrate along vessels to numerous organs ? in domestic equids, the
brain, lymph nodes and kidneys are commonly infected.2
Less than ten cases of human halicephalobiasis have been reported,
making this a very uncommon disease in humans. Halicephalobiasis in humans
manifests as encephalitis and myelitis, and cases have been invariably fatal,
due to not only the non-specific signs of disease but also the unfamiliarity of
human physicians with this parasite.8
In humans, neurological disease associated with helminth infection may occur
with a number of nematodes, including Toxocara canis, Angiostrongylus
cantonensis, Baylisascaris procyonis, Strongyloides stercoralis, and
nematodes lesser known to veterinarians, such as Gnathostoma spinigerum
and Lagochilascaris minor.8 Humans are atypical
hosts for Gnathostoma sp., which utilizes a copepod of the genus Cyclops
as a primary intermediate hosts and a wide range of vertebrates as second intermediate
hosts. Humans may contract gnathostomiasis from eating undercooked seafood
containing the larval nematodes (Asian swamp eels are a common source in areas
of SE Asia. .As humans are aberrant hosts for this parasite, larva will migrate
through a number of tissues, never completing their life cycle. Lagochiascaris
minor is an ascarids which uses the mouse as an intermediate host and a cat
for a definitive host. Accidental ingestion of undercooked rodent meat may
result in visceral larval migrans in the human.1
References:
1. Campos DMB, Barbosa AP, De Oliveira JA, Tavares GG, Cravo PVL, Ostermayer AL. Human lagochilascariasis ? a rare helmintic diseas. PLoS Neglect Trop Dis; s11(6): e0005510.
2. Cantile C, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer?s Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA: Elsevier; 2016:390.
3. Enemark HL et al. An outbreak of bovine menigoencephalomyelitis with identification of Halicephalobus gingivalis. Vet Parasitol 2016; 218: 82-86.
4. Gardiner CH, Poynton SL. Morphologic characteristics of rhabditoids in tissue section. In: An Atlas of Metazoan Parasites in Animal Tissues. Washington DC, AFIP Press, pp. 14-16.
5. Henneke C et al. The distribution pattern of Halicephalobus gingivalis in a horse is suggestive of a haematogenous spread of the nematode. Acta Vet Scand 2014; 56: 1-4.
6. Jung JY et al. Meningoencephalitis caused by Halicephalobus gingivalis in a Thoroughbred gelding. J Vet Med Sci 2014; 76(2): 281-284.
7. Lim CK et al. First human case of fatal Halicephalobus gingivalis meningoencephalitis in Australia. J Clin Microb 2015; 53(5): 1768- 1774.
8. Papadi B, Boudreaux C. Tucker JA, Mathison B, Bishop H, Eberhard ME. Case report Halicepahlobus gingivalis: a rare cause of fata meningoencehalomyelitis in humans. Am J Trop Med Hyg 2013; 88(6):1062-1064.
9. Wilkins PA et al. Evidence of Halicephalobus delatrix (H. gingivalis) from dam to foal. J Vet Intern Med 2001; 15: 412-417.