Signalment:
Gross Description:
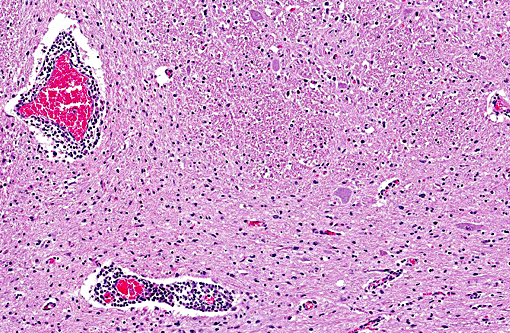
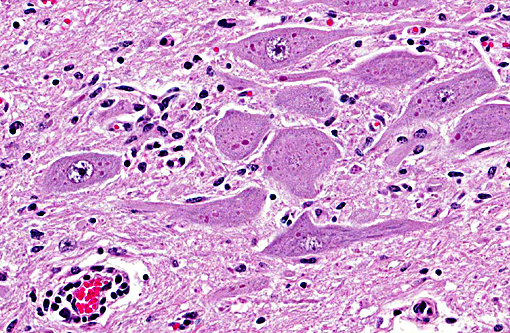
Histopathologic Description:
Morphologic Diagnosis:
Etiology: Lyssavirus of rabies
Condition:
Contributor Comment:
Texas is setting records in rabies cases with several hundred cases reported in the counties adjacent to our laboratory. Because of baited vaccination of wildlife, we have now got less fox and coyote rabies and lots of skunk rabies.(7) Nonhematophagous bats are also commonly infected.(1)
The history was classic(6), and being forewarned, all precautions were taken in taking samples to confirm rabies, as well as completing a complete necropsy. While papers about the virtues of immunohistochemical (IHC) techniques are touted, we must conform to the use of our States official test that is a direct immunofluorescent (DIF) test on chilled brain. That may be a good thing in view of a comparison of diagnostic tests on 26 naturally infected cows in Brazil where IHC detected only 92.4% of cases.(6) The official sample in Texas is a transverse section of the brain extending caudally from the colliculi to the level of and including the midcerebellar cortex. Previous sampling used a midsagittal half of the entire brain, or half of a transverse section of the brain at the level of the cerebellar roof nuclei and the hippocampus (This allowed us to store the other half of these sections in the event of loss of samples.). The previous sites were based on the belief that the cerebellum was best for ruminants while the hippocampus was best for other species. Our State lab has had rare cases where the brain was positive on one side; therefore, we now include transverse pieces of entire brainstem. Otherwise, we will not get an official negative test. Our greatest liability challenges are not dealing with positive cases; rather, things get exciting with getting neither negative or positive results due to improper sampling or sample submission.
Historically, diagnosis of rabies has involved a variety of tests. Direct immunofluorescence, direct immunohistochemistry, mouse inoculation and the demonstration of meningoencephalitis with Negri bodies have been used. A recent comparison of techniques in diagnosis of bovine rabies supported the belief that the cerebellum was an area of 100% positivity with mouse inoculation and direct immunofluorescence; however, the pons, spinal cord and thalamus were also good. Negri bodies were seen 82% of the time in the cerebellum.(4) Similar results were found in a second study.(2) A recent immunohistochemical study of archival tissues of rabies in a variety of species concluded the best site for rabies virus detection in dogs and cats was the hippocampus, but in cattle, viral antigen was most prominent in the brainstem, followed by the cerebellum. In horses, the cervical spinal cord and adjacent brainstem were the optimal sites for detecting rabies virus antigen. In raccoons and skunks, labeling was dispersed more widely; thus, tissue site selection might be less important for these wildlife reservoir species.(9)
It is always difficult to compare results using different techniques conducted in different laboratories, but I hope whoever examines tongue tone in cattle showing the sign of this cow wears proper PPE!
JPC Diagnosis:
Conference Comment:
Rabies virus testing was discussed during the conference in the context of working in areas of the world where high ambient tem-peratures and lack of refrigeration may present challenges that make the fluorescent antibody test less feasible, and the use of other methods such as immunohisto-chemistry (IHC) more practical. However, as mentioned above by the contributor, the accuracy of IHC can be less than 100%; nonetheless, some studies have shown the accuracy of IHC to be very similar to fluorescent antibody testing, indicating it may be a feasible alternative in some cases. Important considerations for IHC include the species of mammal being tested in regard to the area of brain sampled as discussed above, the type of antibody used (polyclonal vs. monoclonal) and the time the tissue remained in formalin.(9) Reverse transcription PCR for rabies diagnosis has been developed and has been shown to have similar results when compared with the fluorescent antibody test;(3) and in some cases has been shown to be superior when there is significant decomposition in the sample.(5)
Besides more commonly implicated wildlife species such as foxes, skunks and raccoons, bats are also an important wildlife rabies reservoir, capable of transmitting the virus to domestic species and humans. Bats are one of the most commonly infected wildlife species in Texas and were indeed the most commonly infected species between 2006 and 2010.(4) A great deal is unknown regarding the complex pathogenesis of rabies in bats, and although uncommon, adaption of bat rabies virus variants into other mammals has occurred.(4) Bats may be exposed to the virus multiple times in their lives, via different routes, which can influence outcome in future exposures. Clinical outcome can vary significantly in infected bats, from rapid clinical progression to survival for several months after infection, and route of infection may play a role in how the disease progresses and the bats ability to transmit the disease.(2) Rabies remains an important public health problem in the United States and worldwide; understanding testing methodology and pathogenesis, in order to facilitate prompt diagnosis in cases of human exposure and to help control infection in domestic species, remains important for public health officials and pathologists alike.
References:
1. Blanton JD, Palmer D, Dyer J, Rupprecht CE. Rabies surveillance in the United States during 2010. J Am Vet Med Assoc. 2011;239:773-83.
2. Davis AD, Jarvis JA, Pouliott CE, et al. Susceptibility and pathogenesis of little brown bats (Myotis lucifugus) to heterologous and homologous rabies viruses. J Virol. 2013;87(16):9008-15.
3. Dupuis M, Brunt S, Appler K, et al. Comparison of automated quantitative reverse transcription PCR and direct fluorescent antibody detection for routine rabies diagnosis in the United States. J Clin Microbiol. 2015;53(9):2983-9.
4. Mayes BC, Wilson PJ, Oertli EH, et al. Epidemiology of rabies in bats in Texas (2001-2010). J Am Vet Med Assoc. 2013;243(8):1129-1137.
5. McElhinney LM, Marston DA, Brookes SM. Effects of carcass decomposition on rabies virus infectivity and detection. J Virol Methods. 2014;207:110-113.
6. Pedroso PM, Leal JS, Dalto AG, Oliveira LGS, Driemeier D. Bovine rabies diagnosed in the Veterinary Pathology Department of the Universidade do Rio Grande do Sul, Porto Alegre, RS, Brasil from 2002 to 2007. Acta Scientiae Veterinariae. 2010;40(1):1015-20.
7. Shwiff SA, Kirkpatrick KN, Sterner RT. Economic evaluation of an oral rabies vaccination program for control of a domestic dogcoyote rabies epizootic: 19952006. J Am Vet Med Assoc. 2008;233:1736-41.
8. Silva MLCR, Riet-Correa F, Galiza GJN, Azevedo SS, Afonso JAB, Gomes AAB. Distribution of rabies virus in the central nervous system of naturally infected ruminants. Pesq Vet Bras.2010;30:940-944.
9. Stein LT, Rech RR, Harrison L, Brown CC. Immunohistochemical study of rabies virus within the central nervous system of domestic and wildlife species. Vet Pathol. 2010;47(4):630-636.
10. Summers BA, Cummings JF, de Lahunta A. Veterinary Neuropathology. St Louis, MO:Mosby; 1995:95-100.