Signalment:
Gross Description:
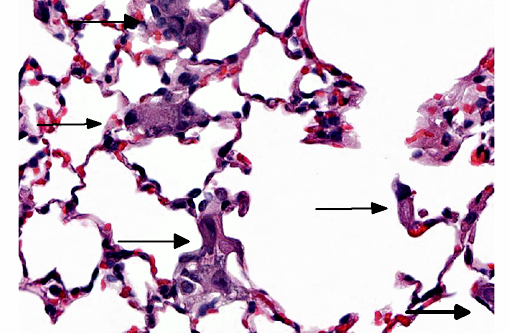
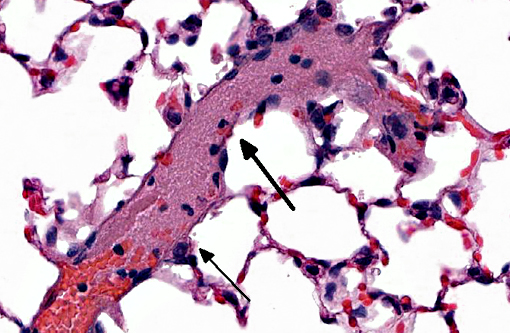
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Nonthrombotic pulmonary embolism is defined as embolization of the pulmonary circulation by different cell types (adipocytes, hemopoietic, amniotic, trophoblastic, or tumor), bacteria, fungi, foreign material, or gas.(4) The pathogenesis of nonthrombotic pulmonary embolism is more complex and diverse than that associated with pure mechanical obstruction of pulmonary embolism caused by vascular thrombi, and as such, often results in unusual or peculiar clinical signs.(4) However, many cases of nonthrombotic pulmonary embolism (regardless of cause) are associated with the development of severe peracute/acute inflammatory reactions in the pulmonary circulation, such as acute respiratory distress syndrome.(4)
Trophoblastic pulmonary embolism refers to embolization of the pulmonary circulation by trophoblastic cells derived from placental tissue.(4) Trophoblastic pulmonary embolism has been documented as a spontaneous condition in mammals with hemochorial placentation (where there is direct contact between fetal trophoblastic cells with the maternal circulation), primarily in humans and chinchillas.(3,4) In these species during pregnancy, syncytiotrophoblasts regularly desquamate from the placenta and into the maternal circulation.(3,4) The intravascular trophoblastic cells usually lodge in capillaries of the alveolar septae of the lung. Histologically, they appear as large (>50-100 μm diameter), round to deformable cells with abundant cytoplasm containing multiple, large (>25-50 μm diameter) nuclei.(3,4) The syncytiotrophoblasts are ultimately removed without consequence.(3,4) Trophoblastic pulmonary embolism is only associated with clinical disease is rare cases, and when it does occur, severe respiratory distress is the most common presentation. Positive antemortem clinical diagnosis is difficult. Most cases of trophoblastic pulmonary embolism diagnosed on microscopic examination of lung is usually incidental.(2,3)
In this case, this mouse was part of an experiment studying the interactions of trophoblasts with the circulation. The trophoblasts are smaller than those noted in spontaneous cases in humans or chinchillas. Their morphology is more similar to cytotrophoblasts rather than syncytiotrophoblasts, and might be explained by the cultured history of the cells. Regardless of morphology, the death of the animal immediately post-injection is most suggestive that rapid trophoblastic pulmonary embolism is the cause of death in this animal.
JPC Diagnosis:
Conference Comment:
While this condition is most often observed in humans and chinchillas, recently it has been described as a common finding in a colony of cotton rats used to study a variety of infectious agents, indicating the possibility of an alternative model of aberrant trophoblastic deportation in women.(4)
In a developing blastocyst, trophoblasts are the peripheral cells first located at a single pole. They eventually give rise to the embryonic portion of the placenta, by proliferating rapidly into two distinct cell populations. The inner population is a monolayer of individual cells which are mitotically active and are known as cytotrophoblasts, while the thicker outer layer is composed of continuous multinucleated cells with no cytoplasmic demarcation and are called syncytiotrophoblasts. Cytotrophoblasts continue to proliferate, with the new cells joining the ranks of syncytiotrophoblasts. As syncytiotrophoblasts increase in number, they form vacuoles that coalesce into large spaces known as lacunae. The continued growth of the syncytium erodes the adjacent endometrium and eventually the maternal blood vessels, allowing maternal blood to fill the newly formed lacunae and nourish the developing embryo.(2) With such close association, it follows that syncytiotrophoblasts could be readily introduced into the maternal blood supply causing their embolization as observed in this case.
References:
1. Delmis J, Pfeifer D, Ivanisevic M, Forko JI, Hlupic L. Sudden death from trophoblastic embolism in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2000;92(2):225-227.
2. Gartner LP, Hiatt JL. Color Textbook of Histology. 2nd ed. Philadelphia, PA: W.B. Saunders Company; 2001:480-482.
3. Ilha MR, Bezerra Jr PS, Sanches AW, et al. Trophoblastic pulmonary embolism in chinchillas (Chinchilla laniger). Ciencia Rural, Santa Maria. 2000;30:903-904.
4. Jorens PG, Van Marck E, Snoeckx, et al. Nonthrombotic pulmonary embolism. European Respiratory Journal. 2009;34:452-474.
5. La Perle KM, Green MG, Niewiesk S. Trophoblast deportation to the lungs of cotton rats (Sigmodon hispidus). Comp Med. 2014;64(6):448-455.
6. Mitchell RN. Hemodynamic disorders, thromboembolic disease, and shock. In: Kumar V, Abbas AK, Fausto N. Robbins and Coltran Pathologic Basis of Disease. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005:135-137.