Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 4
September 20th, 2017
CASE II: 16/545 (JPC 4102434).
Signalment: 6 years old, male, Norwegian lundehund, Canis familiaris, dog.
History: The dog had gastrointestinal signs for approximately 5 months. He could be normal for some days, but had recurrent episodes of diarrhea with thin watery and light-colored feces. The appetite was normal, but decreased the last two weeks. At the clinic, a thickened intestinal segment cranial to the pelvis was noted on abdominal palpation. The feces were very light in color, pale brown to almost yellow-white. The consistency was paste-like and it seemed to contain fat. X-ray and ultrasound revealed severely gas-distended and dilated intestines with almost no peristaltic movements. Exploratory laparotomy was performed. The abdominal cavity contained small amounts of yellow clear fluid, and there were no signs of peritonitis. The stomach, duodenum and pancreas were normal. Nearly all of jejunum and ileum, all the way to the ileocecal junction, was characterized by atony and dilation. There were indications of peristaltic movements, but no proper contraction of intestinal wall muscle. The cecum was small and firm and the colon had near normal diameter. No physical obstruction could be detected, and it was possible to press intestinal contents from the ileum to the colon during the explorative laparotomy. The clinical tentative diagnosis was a condition that affected the function of gut motility, a pseudo-obstruction. There were no other signs consistent with dysautonomia. The dog was euthanized.
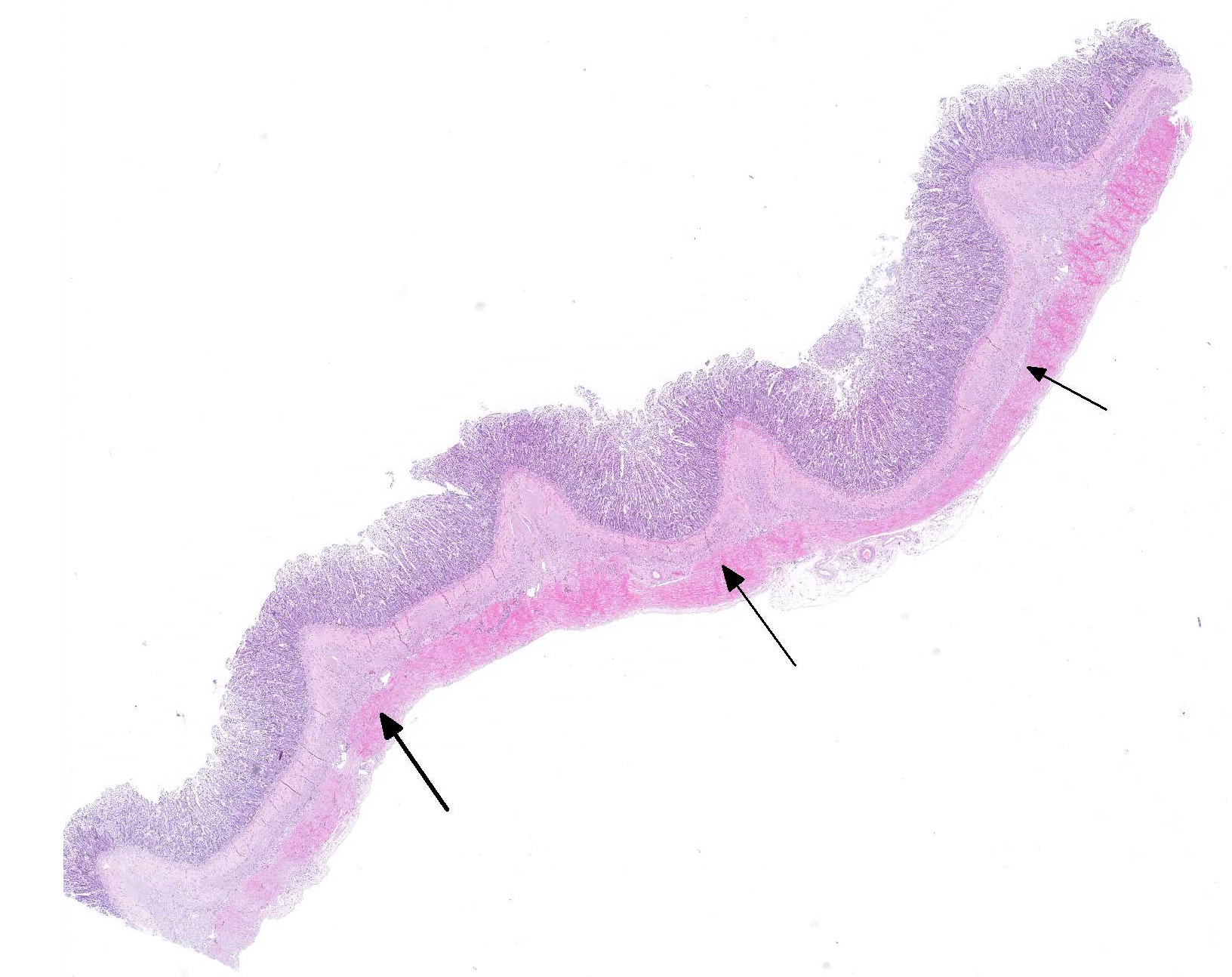
Gross Pathology: The body condition of the cadaver was below normal. The small intestine was severely dilated with abundant light grey-brown content with a thin porridge-like consistency. The content of the colon was similar in color, but a little firmer. There was no hyperemia in the intestinal mucosa, and the surface of the mucosa was also otherwise normal (the breed is predisposed to intestinal lymphangiectasia).
Laboratory results: None provided.
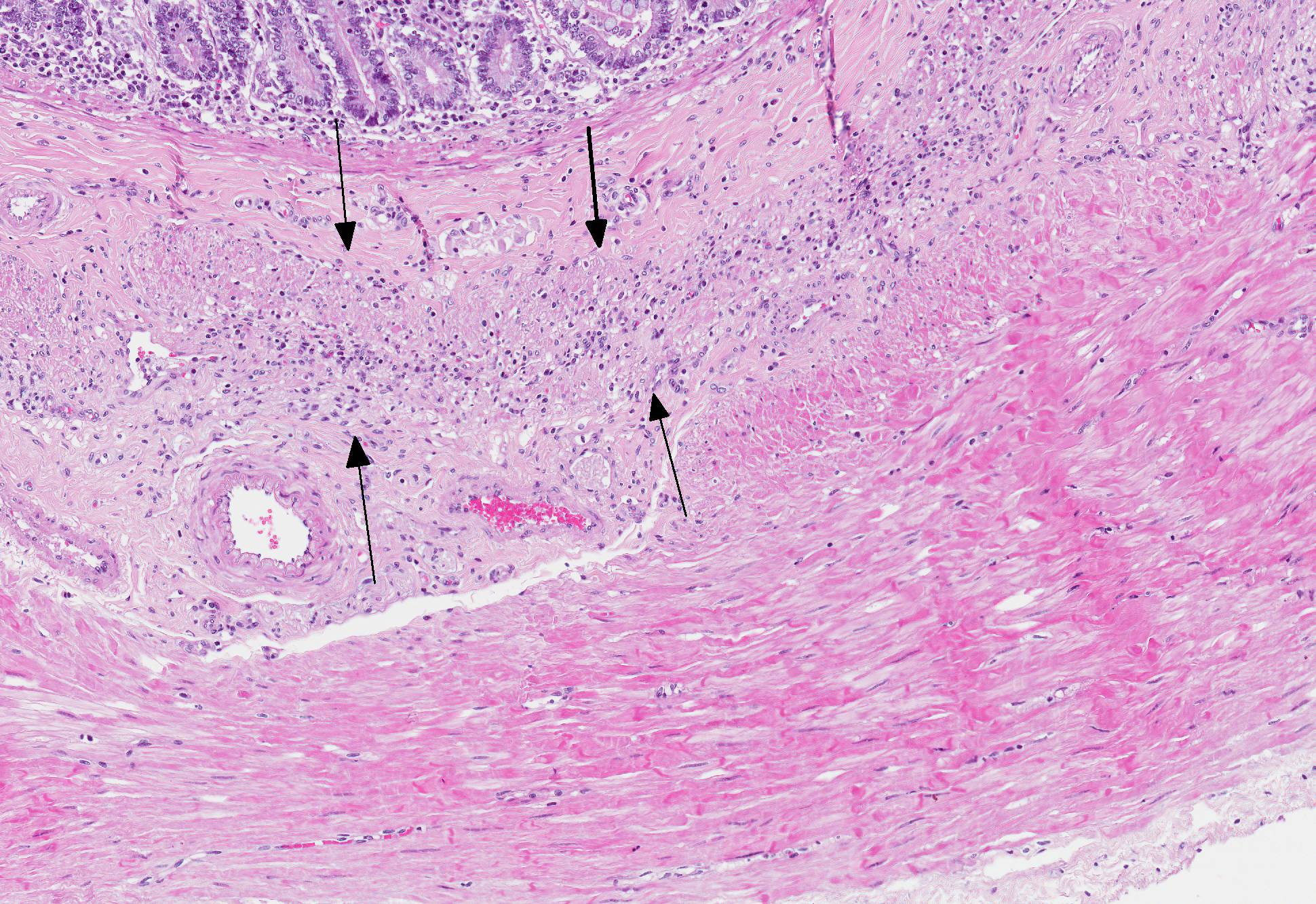
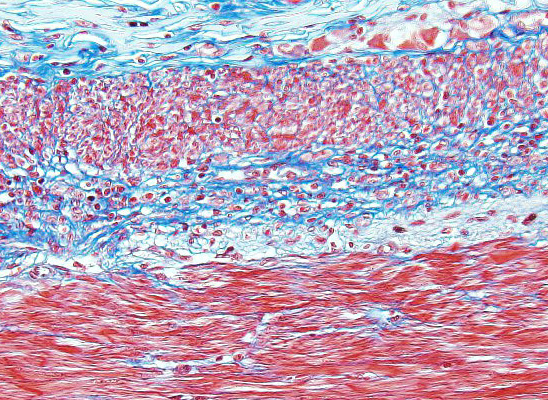
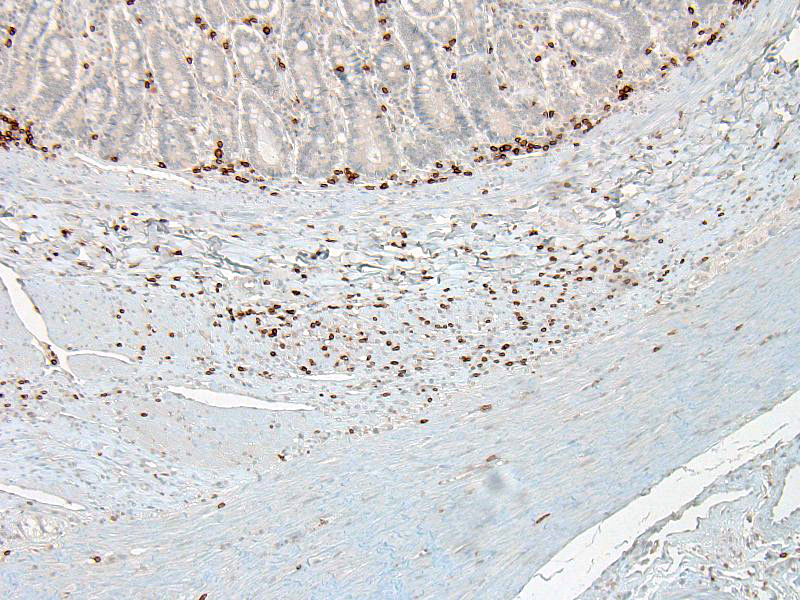
Microscopic Description: In the inner circular layer of the lamina muscularis of the jejunum there is a multifocal to diffuse loss of smooth muscle cells due to degeneration and necrosis. This layer is affected to a variable degree, but usually the innermost part of the lamina muscularis is better preserved and the outer part, towards the Auerbachs plexus and the outer longitudinal muscle, was more severely affected. In affected areas, there was a mild multifocal inflammatory cell infiltrate composed lymphocytes, some plasma cells and histiocytic cells. In the most severely affected areas, there was near total loss of the inner circular layer of smooth muscle cells, and replacement by mild fibrosis. Except for moderate autolytic changes in the mucosa, the small intestine was otherwise normal. The lamina muscularis in stomach and colon was normal.
Contributors Morphologic Diagnosis: Jejunum: Leiomyositis, lymphocytic, chronic with smooth muscle degeneration and necrosis.
Contributors Comment: Chronic intestinal pseudo-obstruction (CIPO) is a syndrome characterized by gastrointestinal dilation without any physical occlusion of the lumen.4 It is a rare condition in humans, and dogs, and single cases are also described in a cat and a horse.1,2,3,5 Morphologically CIPO may be classified as neuropathy, mesenchymopathy or myopathy, based on predominant involvement of enteric neurons, interstitial cells of Cajal or smooth muscle cells, respectively.1
In domestic animals, CIPO may occur in congenital agangliosis, other conditions associated with enteric neuronal loss or ganglioneuritis, the systemic dysautonomias and intrinsic disease of intestinal smooth muscle.4
In dogs, CIPO is rare and associated with intestinal leiomyositis. Affected dogs are of variable ages and breeds, they present with acute or chronic signs of vomiting, regurgitation and small bowel diarrhea.5
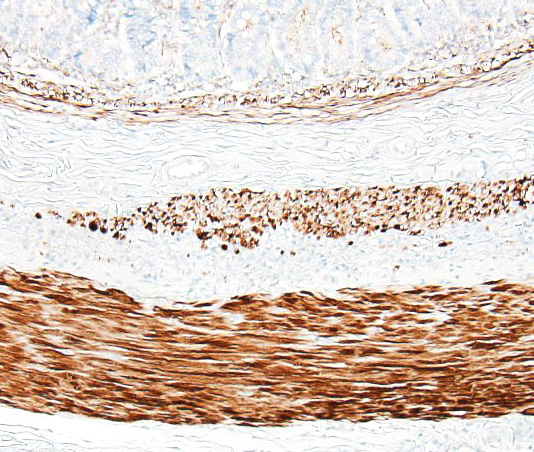
The pathogenesis is unknown, but an autoimmune inflammatory reaction affecting the intestinal lamina muscularis is suspected. Histopathology of small intestine reveals mononuclear inflammation, smooth muscle degeneration and necrosis, and fibrosis centered on areas of myofiber loss. Immunohistochemically, the lymphocytic infiltration is dominated by T lymphocytes, with fewer B lymphocytes. The intestinal lesions may be segmental in early stages, but in chronic and severe cases, there may be near full thickness loss of smooth muscle cells in the lamina muscularis.5
Similar lesions, but milder may also be seen in gastric or colonic wall. In living dogs, a full-thickness intestinal biopsies is required to make a definitive diagnosis.5
JPC Diagnosis: Small intestine, inner circular layer: Leiomyositis, chronic, lymphocytic, diffuse, severe with smooth muscle loss and fibrosis, Norwegian lundehund, canine.
Conference Comment: This is a nice example of chronic intestinal pseudo-obstruction, a rare condition that is described most often in dogs, and results from segmental or diffuse neuromuscular dysfunction leading to a flaccid and dilated section of intestine with no physical obstruction.
In domestic animals, there are two main types of pseudo-obstruction: disorders that affect the ganglia of the myenteric plexi and those that affect the tunica muscularis. In dogs, infiltration of the tunica muscularis with inflammatory cells (predominately T-lymphocytes) and resulting fibrosis is the most common presentation (seen in this case).6
With regard to neurpathic entities, in horses, particularly white foals born of parents with frame overo color patterns (white on both sides of their bodies), the myenteric plexi of the terminal ileum, cecum, and colon are affected in a congenital condition known as congenital colonic aganglionosis or lethal white foal syndrome. Foals with this congenital abnormality are missing the ganglia within those regions of the intestine leading to fatal colic. The gene mutation observed in horses, rodents, and humans with this condition is a loss of function mutation of the endothelin receptor type B gene. This gene functions in timing of the migration of cells of the neural crest. In addition to the myenteric plexus, these foals are also lacking melanocytes in the skin (also derived from the neural crest) which explains their white color. A similar genetic condition of Clydesdale foals is associated with hypoganglionosis of the myenteric plexus resulting in megacolon. The pathogenesis in this unknown, although it does occur in older foals (4-9 months old) indicating an acquired condition.6
Dysautonomia or Key-Gaskell syndrome was briefly discussed as a rare entity affecting cats under 3 years of age with an unknown pathogenesis. This syndrome presents as disordered motility, with affected animals that often die due to regurgitation, prolonged starvation, or aspiration pneumonia. Affected neurons in the cranial nerve nuclei III, V, VII, and XII, ventral horns of the spinal cord and dorsal root ganglia appear chromatolytic on light microscopy. Ultrastructurally they have a characteristic appearance with autophagocytic vacuoles, dilated cisternae, and stacks of smooth endoplasmic membranes in their cytoplasm.6 Lastly, proventricular dilatation disease (PDD) was reviewed caused by avian bornavirus. PDD causes flaccidity and dilation of any portion of the gastrointestinal tract in parrots, macaws, conures, and cockatoos due to lymphoplasmacytic ganglioneuritis of the myenteric plexi resulting in atrophy of the intestinal wall.4
The main differential myopathic condition discussed was canine immune-mediated polymyositis which may involve muscle damage by T-lymphocytes within the alimentary tract, particularly skeletal muscle of the esophagus. Polymyositis is overrepresented in German Shepherd Dogs and Newfoundlands and may occur as part of a spectrum of disease along with systemic lupus erythematosis which is diagnosed by a positive antinuclear antibody (ANA) titer. Testing for serum antibodies to type 2M myosin may aide in diagnosis of polymyositis because most affected dogs lack serum antibodies to 2M myosin.3 In this case, immune-mediated polymyositis is not likely the cause since it is the smooth muscle in the tunica muscularis that is affected in this dog.
References:
1. Antonucci A, Fronzoni L, Cogliandro L, et al. Chronic intestinal pseudo-obstruction. World J Gastroenterol. 2008;14:2953-2961.
2. Chenier S, Macieira SM, Sylvestre D, Jean D. Chronic pseudo-obstruction in a horse: A case of myenteric ganglioneuritis. Can Vet J. 2011;52:419-422.
3. Cooper BJ, Valentine BA. Muscle and tendon. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals, Vol 1, 6th ed. St. Louis, USA: Elsevier; 2016:227-228.
4. Harvey AM, Hall EJ, Day MJ, Moore AH, Battersby IA, Tasker S. Chronic intestinal pseudo-obstruction in a cat caused by visceral myopathy. J Vet Intern Med. 2005;19:111-114.
5. Schmidt RE, Reavill DR, Phalen DN. Gastrointestinal system and pancreas. In: Pathology of Pet and Aviary Birds. 2nd ed. Ames, IA: John Wiley & Sons, Inc.; 2015:69.
6. Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals, Vol 2, 6th ed. St. Louis, USA: Elsevier; 2016:74, 77-78.
7. Zacuto AC, Pesavento PA, Hill S, et al. Intestinal leiomyositis: A cause of chronic intestinal pseudo-obstruction in 6 dogs. J Vet Intern Med. 2016;30:132-140.