Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 24
10 April, 2019
CASE IV: 16L-2013 (JPC 4085442)
Signalment: 1 year 5 month old female neutered Newfoundland (Canis lupus familiaris) dog.
History: The animal presented to the Department of Veterinary Neurology at the University of Liverpool’s small animal teaching hospital with a history of chronic right pelvic limb monoparesis and lameness that had significantly worsened in the last 2-3 weeks prior to presentation along with severe weight loss. The referring veterinary surgeon had performed CBC and biochemistry which were unremarkable; gracillis muscle biopsy that revealed muscle atrophy of unknown etiology. On clinical examination patella reflex was absent in the right hind limb with mildly reduced withdrawal reflex. Magnetic resonance imaging (MRI) was performed and revealed an infiltrative paravertebral mass and due to the guarded prognosis euthanasia was performed and a full post mortem completed.
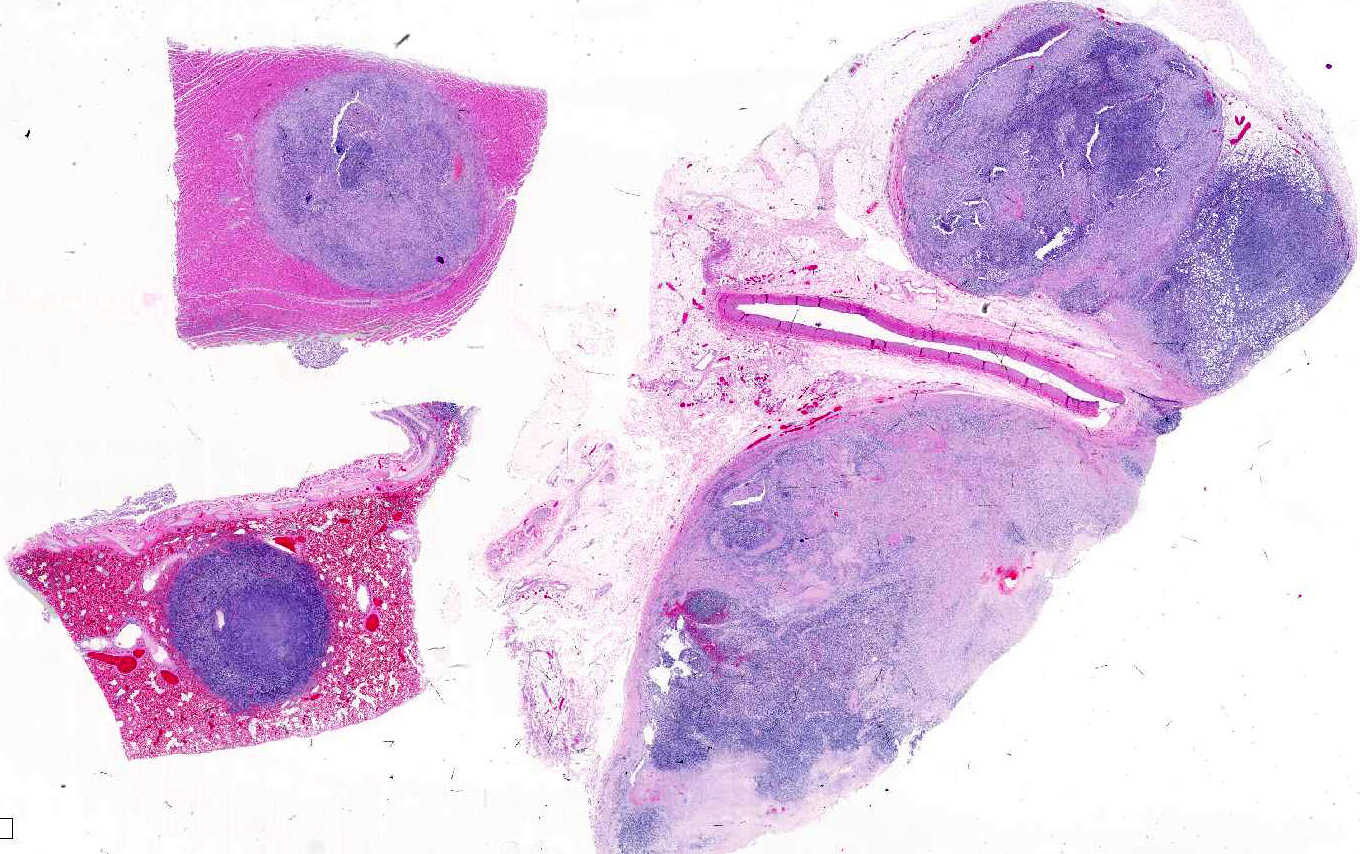
Gross Pathology: There was a large lumbar to sacral paraverterbral multi-lobulated tan firm infiltrative mass within the paravertebral right muscles extending from the level of L4 to the sacrum and expanding into the retroperitoneal space measuring 18 x 6 x 9 cm. The mass invaded the right side of L5-L7 vertebrae. Similar tan masses up to 2 cm in diameter were observed randomly scattered in the liver, pancreas, omentum, lung and myocardium. There was also enlargement of the right ovary by a tan firm mass measuring 7 x 4.5 x 2 cm. Multifocal masses with full thickness ulcerations were observed in the gastric mucosa of the fundic region.
Laboratory results: MRI of lumbar spine: there was a large (18 x 6 x 9 cm) irregularly shaped and multilobulated complex soft tissue mass located in the paraspinal soft tissue ventral to the caudal lumbar spine extending from the level of L4 to the sacrum. The mass filled the right side of the epidural space and wrapped around the cauda equine and the caudal spinal cord. The mass invaded the right side of L5-L& vertebrae. The mass caused ventral displacement of the abdominal organs and compression of the aorta and caudal vena cava.
Cytology: large numbers of neoplastic cells arranged in loose aggregates, moderately cohesive sheets or individualized amid a finely stippled pink background. Occasional macrophages with erythrophagia and intracytoplasmic hemosiderin are scattered throughout. Neoplastic cells are pleomorphic spanning in morphology from small round cells with a high nuclear to cytoplasmic ratio, round nucleus, granular chromatin and scant blue cytoplasm to polygonal cells with more abundant and often vacuolated cytoplasm to clearly fusate cells with oval to reniform nuclei sometimes embedded amid scant pink extracellular matrix and occasionally surrounded by capillaries. Nucleoli are consistently large and pale blue, in number of one to three. Mitoses are occasionally seen. Rarely, small variably sized round dark blue granules (possible hemosiderin) are noted within the cytoplasm. Occasionally, erythrocytes are closely associated with these cells or a single erythrocyte is seen intracellularly. Anisocytosis and anisokaryosis are moderate. Rare elongated multinucleated cells are noted (either neoplastic cells or (‘strap cells’). In one slide small fragments of skeletal muscle myocytes with occasional centralized nuclei are noted scattered amid the neoplastic cells.
Cytological findings are most suggestive of a sarcoma possible rhabdomyosarcoma.
Trucut biopsy: Focally within the section, cells arranged in linear streams within a moderate amount of collagenous stroma, are elongated with indistinct cell borders, variably sized from 40 x 20 μm up to 300 x 200 μm with abundant pale to brightly eosinophilic cytoplasm, sometimes containing striations, and multiple, up to 7, central to para-central nuclei. Chromatin is finely stippled to coarsely clumped often with a central prominent nucleolus. Adjacent to this there are extensive to polygonal, with indistinct cell borders, approximately 30 x 10 μm with abundant eosinophilic cytoplasm and a central elongated vesiculated nucleus. Chromatin is finely stippled and nucleoli are often indistinct. There is scant fibrovascular stroma and scattered necrotic cells. Mitotic figures are rare. There is mild to moderate multifocal mixed (neutrophils-dominated) inflammation.
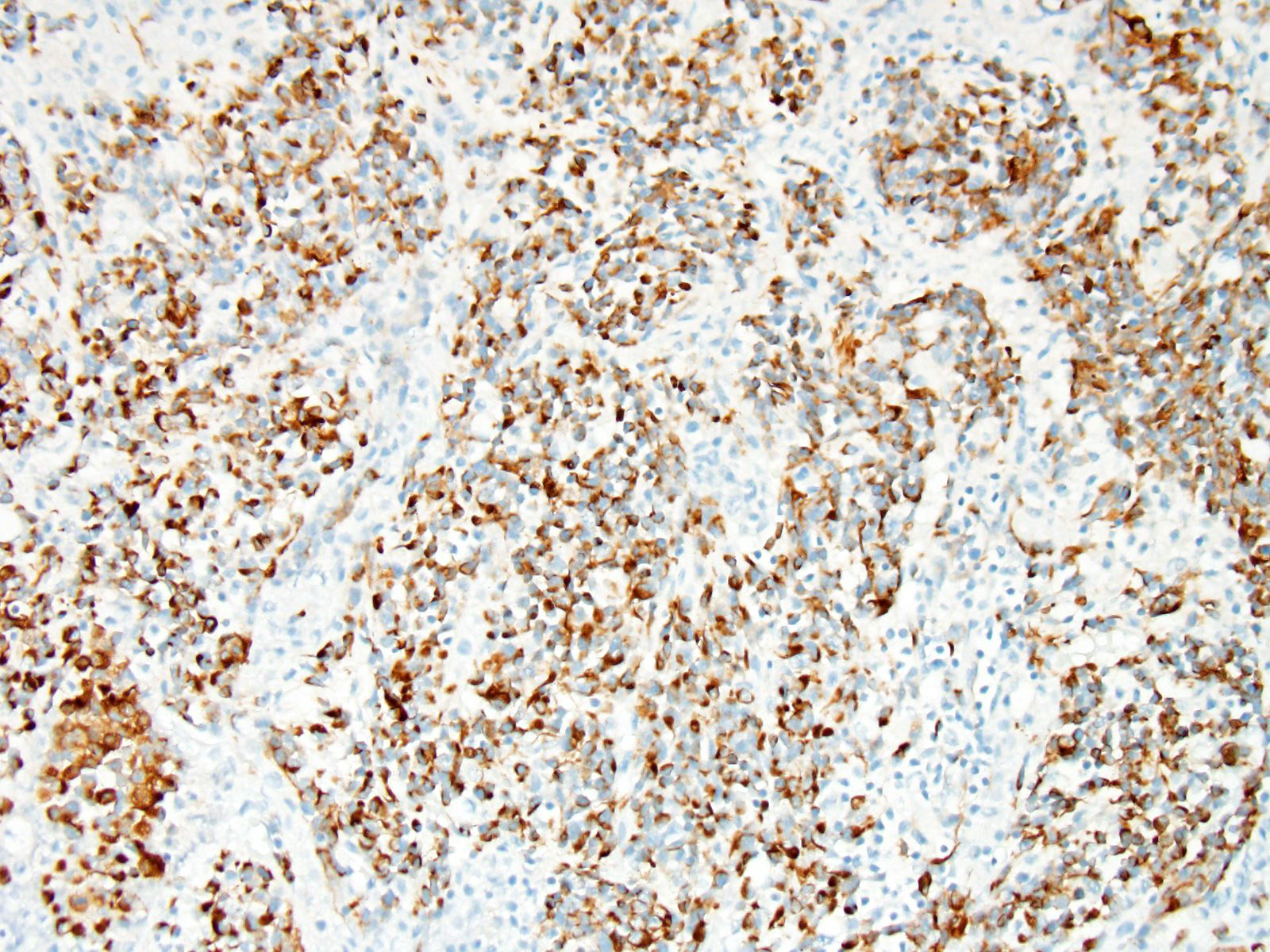
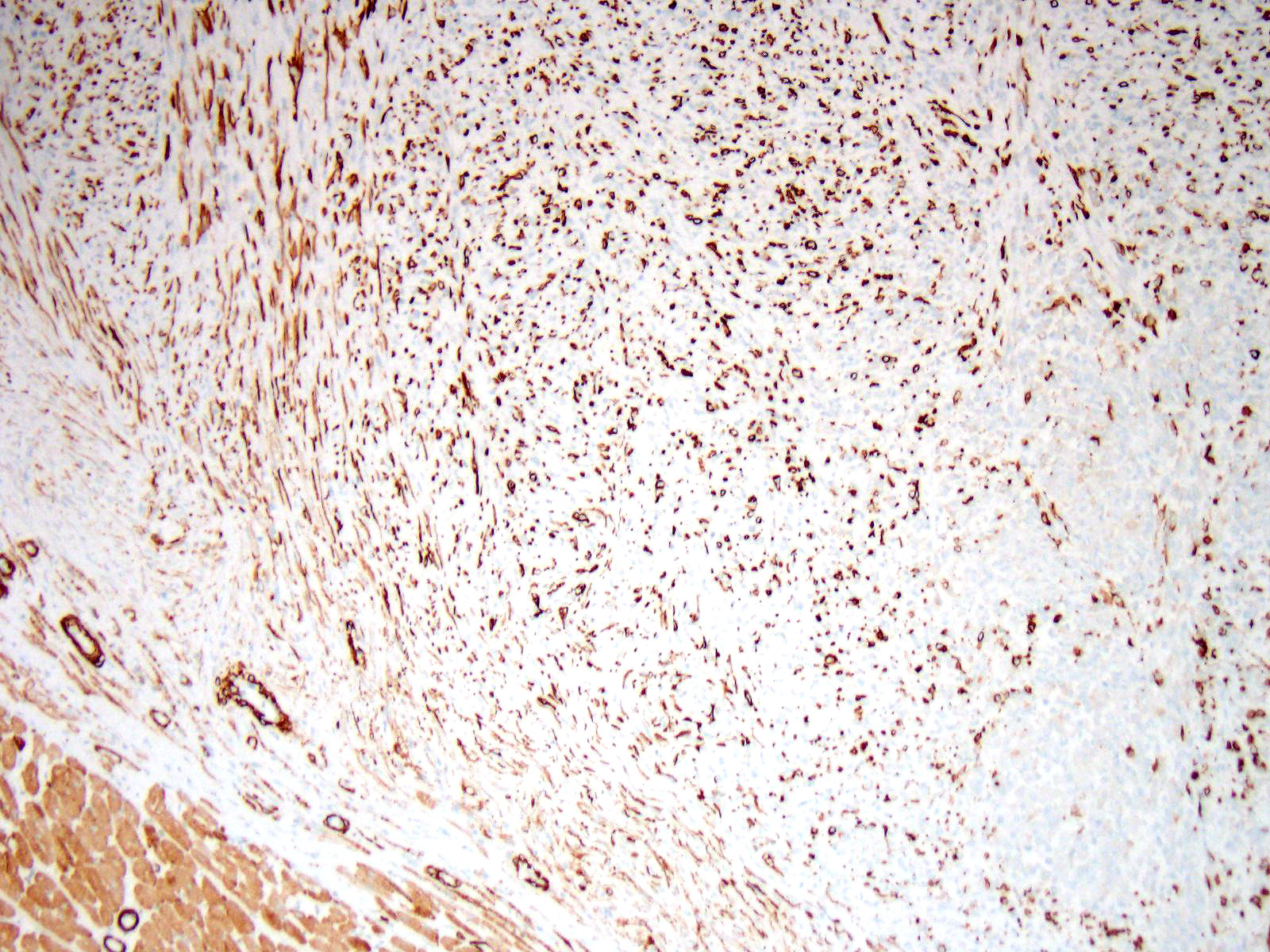
Immunohistochemistry: Immunohistochemistry for pancytokeratin, vimentin and myoglobin was performed on sections of the skeletal muscle mass, the mass in the myocardium and on the mass in the lung. The neoplasm was strongly positive for vimentin and negative for pancytokeratin. There were approximately 40% of neoplastic cells that were positive for myoglobin.
Microscopic Description:
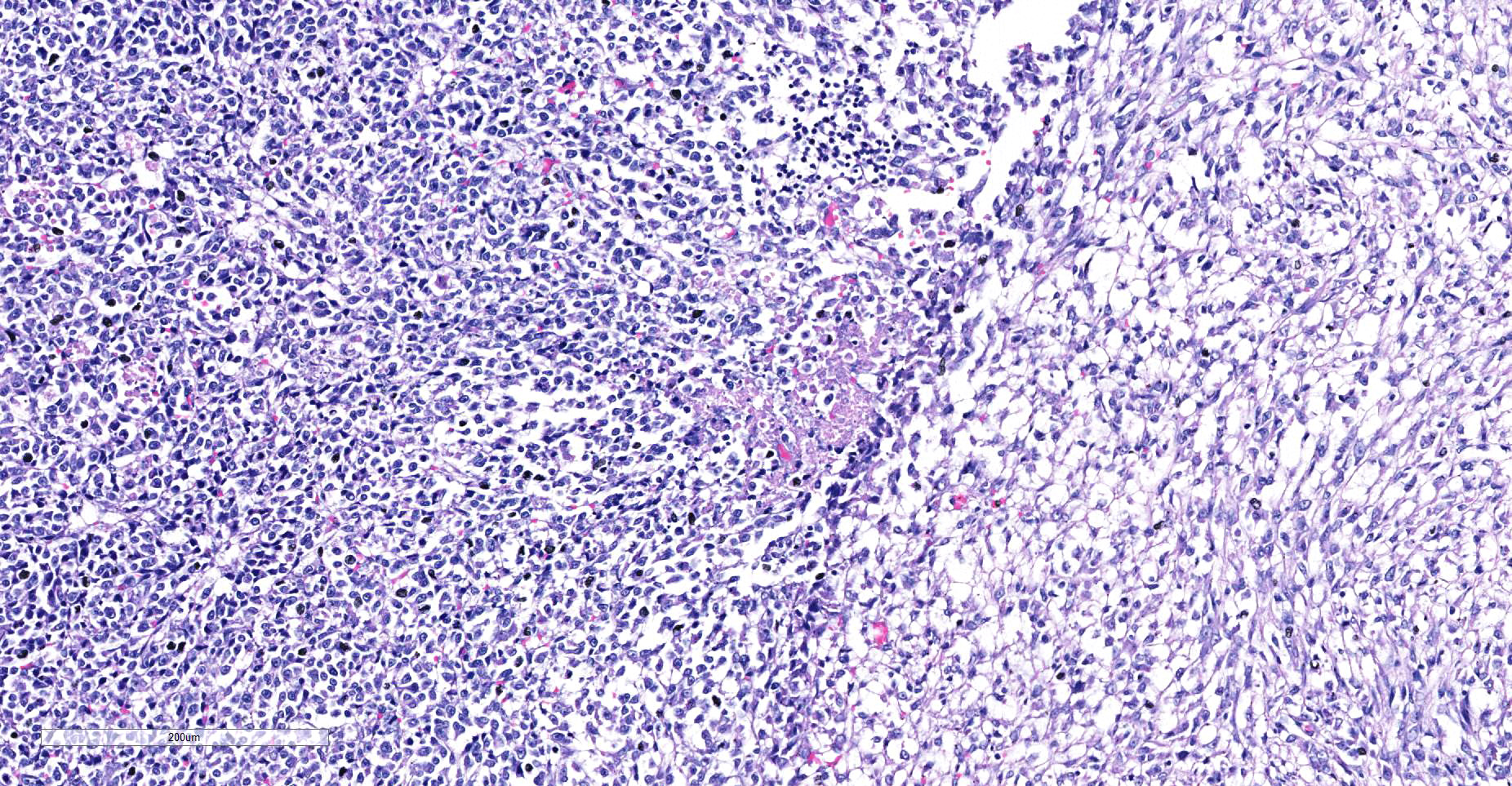
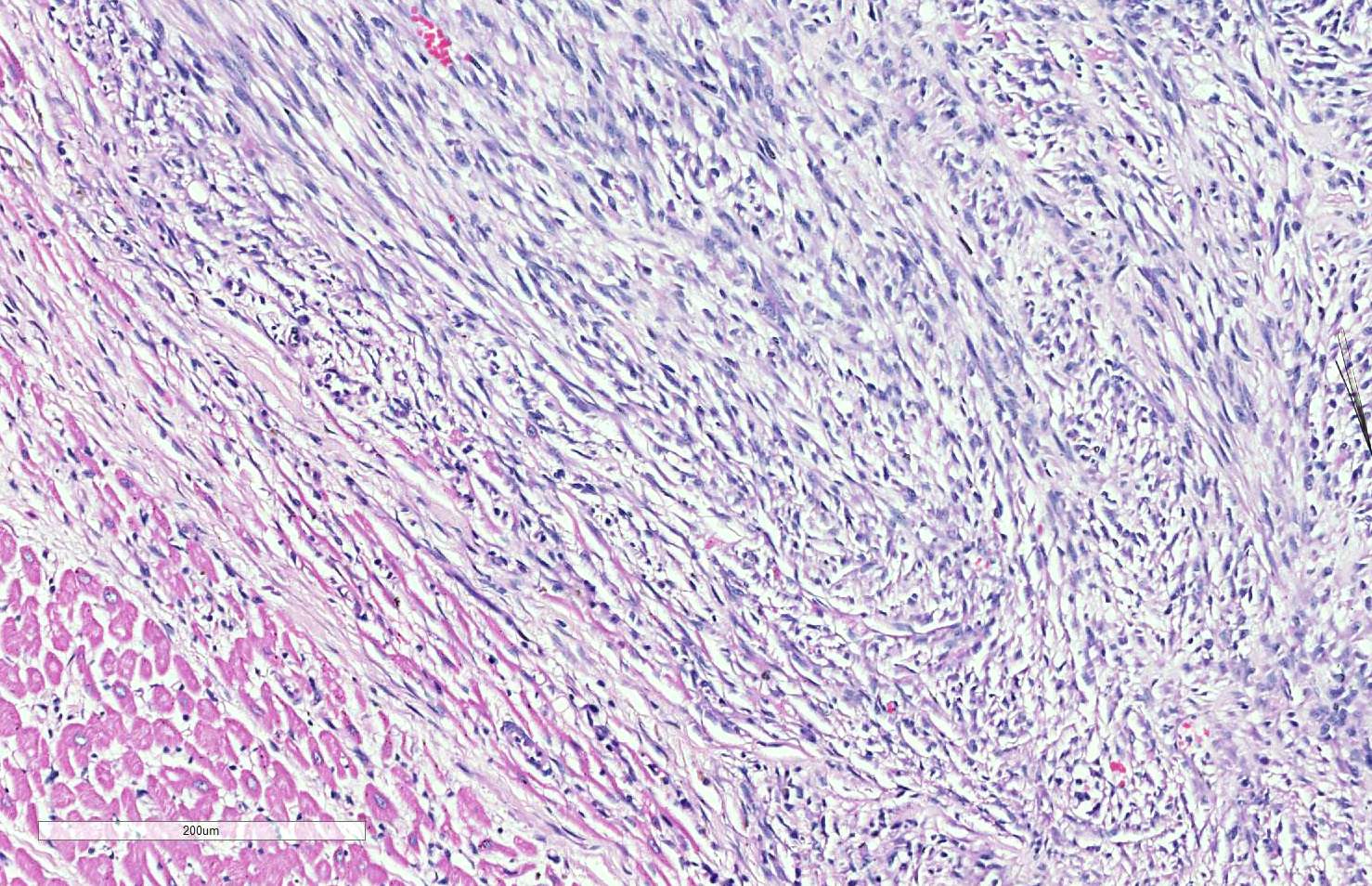
Skeletal muscle: Completely effacing the skeletal muscle there is a non-encapsulated, poorly demarcated, densely-cellular and infiltrative neoplasm. The neoplasm is biphasic with one population of cells arranged haphazardly into streams and the second population arranged into sheets. Cells of the first population are spindled with indistinct cell borders, approximately 15-40 µm with abundant pale eosinophilic cytoplasm and usually a single centrally placed nucleus. Occasionally these cells are large and contain many up to 6 nuclei arranged in a row (strap cell, fig. 4, variable between slides). Chromatin is finely stippled to coarsely clumped and nucleoli vary from indistinct to a prominent single nucleolus to up to 3 nucleoli. Anisocytosis and anisokaryosis are marked and mitotic index is 1 per 10HPF. The second population is interwoven with the first and is composed of sometimes densely packed sheets and other times with a looser arranged alveolar-like structure (fig. 5) with cells sloughed into a lumen both with a fine fibrovascular stroma. Cells are round with mostly distinct cell borders, approximately 20-30 µm diameter with a moderate amount of eosinophilic cytoplasm and a large round to oval central to para-centrally placed nucleus (rhabdomyoblasts). Occasionally cells are multinucleated and several nuclei are aligned in a row. Chromatin is finely stippled and dense to coarsely clumped and open with nucleoli variable from indistinct up to 3 per nucleus. Anisocytosis and anisokaryosis are marked and mitotic index is 6 per 10 HPF. Multifocally throughout the neoplasm there are large areas of necrosis and smaller areas of hemorrhage.
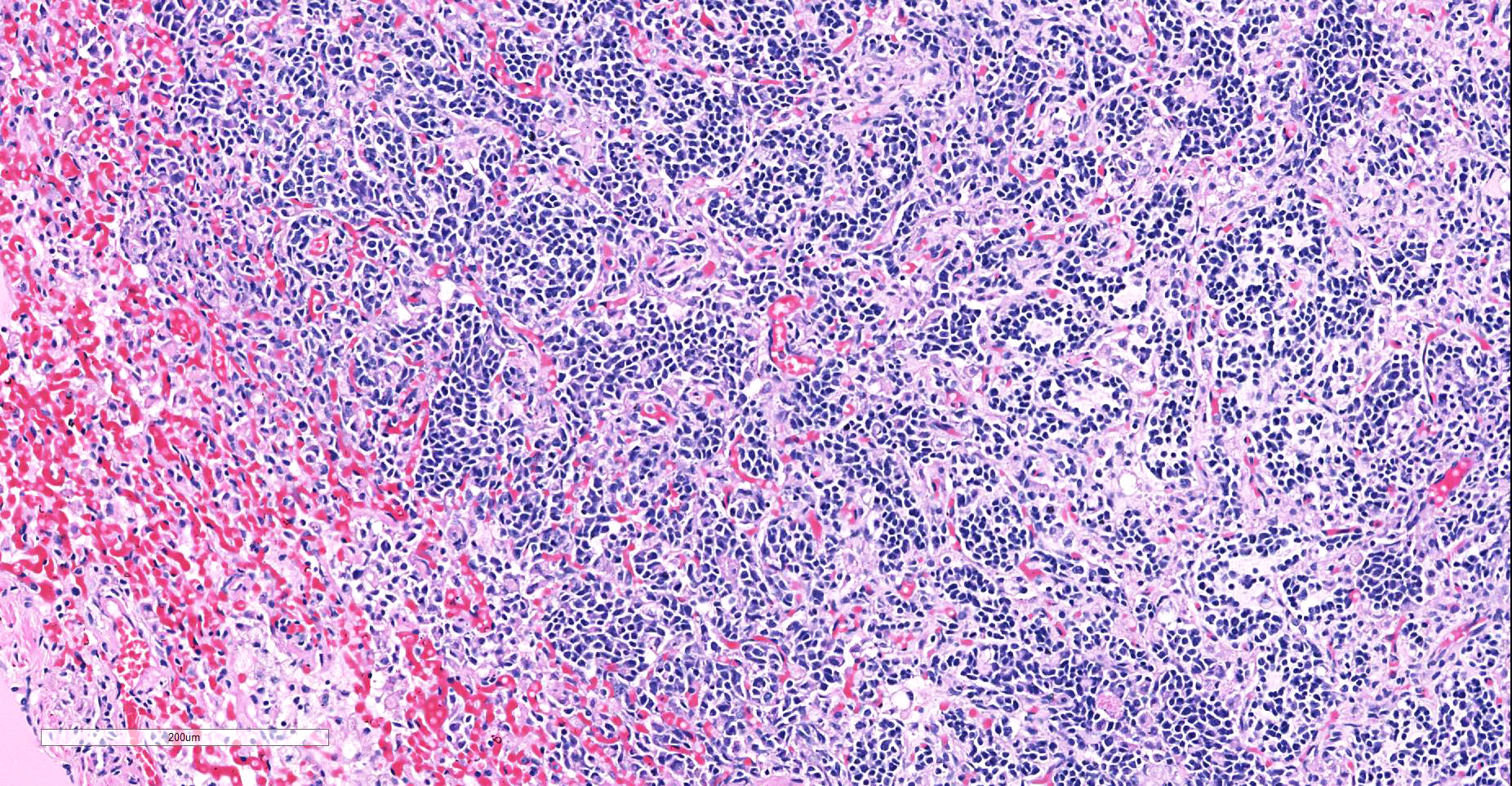
Heart: Within the myocardium there is an approximately 6 mm diameter well demarcated, non-encapsulated densely cellular neoplasm. The neoplastic cells are arranged haphazardly in streams and with a smaller population arranged in sheets with a moderate quantity of fibrovascular stroma. Cells arranged in streams are spindled with indistinct borders, approximately 5 x 30 µm with abundant eosinophilic cytoplasm and an elongated centrally placed nucleus. While cells arranged in sheets are round to polygonal with distinct cell borders, approximately 10-20 µm in diameter with a moderate amount of eosinophilic cytoplasm and a large round to polygonal central to para-centrally placed nucleus. Occasionally in both populations there are large elongated multinucleated cells with nuclei arranged in a row (strap cells, variable between slides). Chromatin is finely stippled to coarsely clumped with from indistinct up to 3 nucleoli per nucleus. Anisocytosis and anisokaryosis are marked and mitotic index is 9 per 10 HPF which are often bizarre.
Multifocally scattered throughout the neoplasm there are areas of lytic necrosis admixed with small amounts of haemorrhage. Also scattered throughout the neoplasm there are areas of deeply basophilic irregular vacuolated material.
Lung: In the lung there is and approximately 5 mm diameter non-encapsulated, reasonably well demarcated but infiltrative, densely cellular neoplasm. The neoplasm is tri-phasic with cells arranged in sheets, streams, cords and packets and sometimes reproducing alveolar-like structures divided by abundant fibrovascular stroma. Cells are hyperchromatic, round to polygonal to spindled with mostly distinct cell borders, approximately 10 µm diameter up to 15 x 30 µm and 10 x 40 µm with a moderate amount of eosinophilic cytoplasm and a central to para centrally placed, round to polygonal to elongated hyperbasophilic nucleus. Chromatin is finely stippled to coarsely clumped and there are indistinct up to 3 nucleoli per nucleus. Cells are highly pleomorphic and mitotic index is 23 per 10 HPF often with bizarre mitotic figures. There are large areas of coagulative necrosis multifocally within the neoplasm and smaller areas of hemorrhage. The lung is diffusely moderately hyperemic.
Contributor’s Morphologic Diagnoses:
Skeletal muscle rhabdomyosarcoma, alveolar type
Heart metastatic rhabdomyosarcoma
Lung metastatic rhabdomyosarcoma
Contributor’s Comment: Rhabdomyosarcoma (RMS) is the malignant neoplasm derived from skeletal muscle. They are highly variable in-terms of age of onset, location, gross and histological appearance1,2,3,4,5,10. RMS are common in human children and juvenile dogs under 2 years old2. Both the human and veterinary RMS can be separated into different categories depending on the various degrees of differentiation towards skeletal muscle.
The veterinary RMS can be subclassified as follows2,4,5,6,7:
- Embryonal RMS: embryonal RMS can be further divided due to the cell morphology into Myotubular, Rhabdomyoblastic and Spindle cell. Another embryonal RMS is the Botryoid embryonal RMS. All embryonal RMS are most frequently encountered in juvenile animals (less than 2 years old).
- Myotubular – this is a RMS that is dominated by classical multinucleated strap cells with frequently observable cross-striations.
- Rhabdomyoblastic – is composed of mostly small round to polygonal cells with abundant eosinophilic cytoplasm and only occasionally cells with cross striations. This may be confused with the solid variant of the alveolar RMS.
- Spindle cell – The RMS is composed of mostly spindle shaped cell in low cellularity often arranged in a storiform pattern.
- Botryoid RMS – RMS that is usually found in the trigone region of the urinary bladder with predominance in the female population and large breed dogs. The term botryoid is used due to the macroscopic grape like appearance. The tumor must be contained in the submucosa.
- Alveolar RMS- the alveolar pattern is determined by the presence of an area of neoplastic cells that are arranged in a glandular like pattern to give “alveolar” spaces. There is also a solid variant where the cells are in dense sheets. Often in the alveolar RMS there will only be a small area displaying the “alveolar” pattern and the rest of the neoplasm is in sheets or anaplastic. Neoplastic cells tend to be round with small to moderate amounts of eosinophilic cytoplasm and multinucleated giant cells (strap cells) that are common in the embryonal RMS are uncommon in the alveolar variant.
- Pleomorphic RMS- rare as only RMS that do not display any features of embryonal or alveolar RMS can be termed truly pleomorphic. The histological features are of a mesenchymal neoplasm with marked anisocytosis and anisokaryosis and bizarre mitotic figures. The pleomorphic RMS tends to be observed in adult rather than juvenile animals.
RMS is one of the most common neoplasms in human children under 15 years old with there being a difference in survival time between embryonal and alveolar variants1,6,7,8,9. It is therefore important to be able to differentiate between the subtypes. The prognostic impact of dividing RMS into subclasses has not yet been determined for veterinary species however in human medicine the pleomorphic and the alveolar variants have the worse prognosis with the embryonal variants having a more favorable outcome6.
Differential diagnosis for RMS are nephroblastoma, germ cell tumors, malignant peripheral nerve sheath tumors and anaplastic sarcomas2,6. It can be extremely difficult to differentiate some RMS on histological cell morphology alone especially if there are low numbers of strap cells. Therefore further diagnostic techniques such as immunohistochemistry and electron microscopy are often required2,4. Due to RMS being a mesenchymal proliferation neoplastic cells label strongly for vimentin. Desmin may be useful but labelling can be uneven in both human and veterinary species. Smooth muscle actin can be used to rule out leimyosarcomas2. Myoglobin expression may be decreased or lost on undifferentiated rhabdomyosarcomas. Immunohistochemistry was performed on our case which was strongly positive for vimentin and many neoplastic cells were positive for myoglobin and all neoplastic cells were negative for pan-cytokeratin.
The case presented here is most likely the alveolar variant of a rhabdomyosarcoma due to the areas within the primary and the metastatic neoplasms having alveoli-like structures. All rhabdomyosarcomas have a degree of pleomorphism and only if there is no evidence of embryonal or alveolar differentiation then the pleomorphic variant can be diagnosed6. A variant described in human medicine but not in veterinary medicine is the spindle cell/sclerosing rhabdomyosarcoma11. The spindle cell/sclerosing RMS is described as being composed fascicles of spindle cells or primitive round cells embedded in sclerotic matrix with varying numbers of rhabdomyoblasts11 which can often mimic the classic alveolar pattern9 and is consistent with some areas in the case presented here.
Clinical features of all RMS vary depending on the location of the neoplasm. Embryonal RMS (except botryoid RMS) are usually observed in the head and neck regions2. Alveolar RMS can be found in a wide range of regions and pleomorphic RMS tend to be within the skeletal muscle2,4.
Contributing Institution:
University of Liverpool, Leahurst Campus, Chester High Road, Neston, Wirral, UK; https://www.liverpool.ac.uk/vetpathology/
JPC Diagnosis: Paraspinal fibrovascular tissue (per contributor): Rhabdomyosarcoma, alveolar type.
Heart, lung: Rhabdomyosarcoma, alveolar type, metastatic
JPC Comment: The contributor has provided an excellent review of the subclassification of rhabdomyosarcoma in human and veterinary species, and in doing so, has illustrated the difficulty so often encountered in trying to classify them. This is also a process that, as mentioned by the contributor, is not of as much importance in veterinary medicine as it is in human medicine, where prognostic information is based on precise classification. .
The neoplasm in this case shows two definitive phenotypic morphologies – that of a spindle cell tumor (myotubular) with few classic strap cells, as well as the nesting and packeting of cells with a polygonal appearance, the so-called alveolar pattern (presence in the lung here notwithstanding. The primary neoplasm of the paraspinal musculature demonstrates both patterns, while in the metastatic nodule in the lung the alveolar pattern predominates, and in the heart, the spindle cell pattern predominates (likely attesting to different microenvironments in various metastatic sites.
A number of immunohistochemical markers were applied on this section, although the morphology of the neoplasm is strongly suggestive of a skeletal muscle, and the metastatic nodules meets the most critical determinant of malignancy. Vimentin was not used in this case due to the obvious spindle cell morphology – quite a number of human pathologists at the JPC eschew the use of vimentin in many subspecialties. The appropriate choice of immunostains in muscle neoplasms may not only indicate a diagnosis, but also yield clues as to the degree of differentiation. Desmin and muscle-specific actin are non-specific muscle markers that will stain smooth and cardiac muscle as well as myofibroblasts. Smooth muscle actin should not stain skeletal muscle but may be an important part of an immunopanel to rule out smooth muscle tumors. Myoglobin is expressed in skeletal and cardiac muscle, but not smooth muscle, and may or may not be expressed in skeletal muscle malignancies, especially poorly differentiated tumors. Myogenin and myoD1 are skeletal muscle-specific nuclear products which are expressed early in development, and may be critical in identifying poorly differentiated neoplasms in veterinary medicine, and have shown utility in differentiating embryonal and alveolar rhabdomyosarcomas in human cases.
The stalwart procedure of identifying cross-striations on a Masson’s trichrome or phosphotungstic acid hematoxylin (PTAH) stain or ultrastructural analysis has largely been supplanted by the use of immunostains and lack of disposable time and patience by today’s veterinary pathologist.
The precise diagnosis in this case was also a subject of lively discussion. Some attendees favored a simple diagnosis of rhadomyosarcoma, suggesting that the biphasic nature of this neoplasm may simply be a factor of sampling (as is often seen in osteosarcoma), or perhaps factors associated with metastatic environment. Ultimately, upon review of gross necropsy findings and current classification (as listed above), the group agrees with the contributor based on the distribution of metastases in multiple tissues as well as the alveolar pattern seem most prominently in the pulmonary metastatic focus.
References:
- Akkoc A, Ozyigit MO, Yilmaz R, et al. Cardiac metastasizing rhabdomyosarcoma in a great Dane. Vet Rec. 2006;156:803-804.
- Caserto BG. A comparative review of canine and human rhabdomyosarcoma with emphasis on classification and pathogenesis. Vet Pathol. 2013;55(5):806-826.
- Ginel PJ, Martín de las Mulas J, Lucena R, et al. Skeletal muscle rhabdomyosarcoma in a dog. Vet Rec. 2002;151:736-738.
- Maxie MG. Jubb, Kennedy, and Palmer’s Pathology of domestic animals. Elsevier. 2016;1(3):241-244.
- Morris JS, Bostock DE, McInnes EF, et al. Histopathological survey of neoplasms in flat-coated retrievers, 1990 to 1998. Vet Rec. 2000;147:291-295.
- Newton WA, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas. 1995;76(6):1073-1085.
- Parham DM. Pathologic classification of rhabdomyosarcomas and correlations with molecular studies. Mod Pathol. 2001;14(5):506-514.
- Parham MD, Barr FG. Classification of rhabdomyosarcoma and its molecular basis. Adv Anat Pathol. 2013;20(6):387-397.
- Rudzinski ER, Anderson JR, Hawkins DS, et al. The World Health Organization classification of skeletal muscle tumors in pediatric rhabdomyosarcoma. Arch Pathol Lab Med. 2015;139(10):1281-1287.
- Yhee J, Kim D, Hwang D, et al. Hematogenous metastasis of embryonal rhabdomyosarcoma originating from skeletal muscle in a young dog. J Vet Diagn Invest. 2008;20:243-246.
- Zhao Z, Yin Y, Zhang J, et al. Spindle cell/sclerosing rhabdomyosarcoma:case series from a single institution emphasizing morphology, immunohistochemistry and follow up. Int J Clin Exp Pathol. 2015;8(11):13814-13820.