WSC 2023-2024, Conference 22, Case 3
Signalment:
10-year-old male neutered dog (Canis familiaris).
History:
Left hind leg/distal femur mass. Radiographs showed bone lysis and periosteal reaction.
An amputated hind leg was received at the diagnostic laboratory for diagnosis.
Gross Pathology:
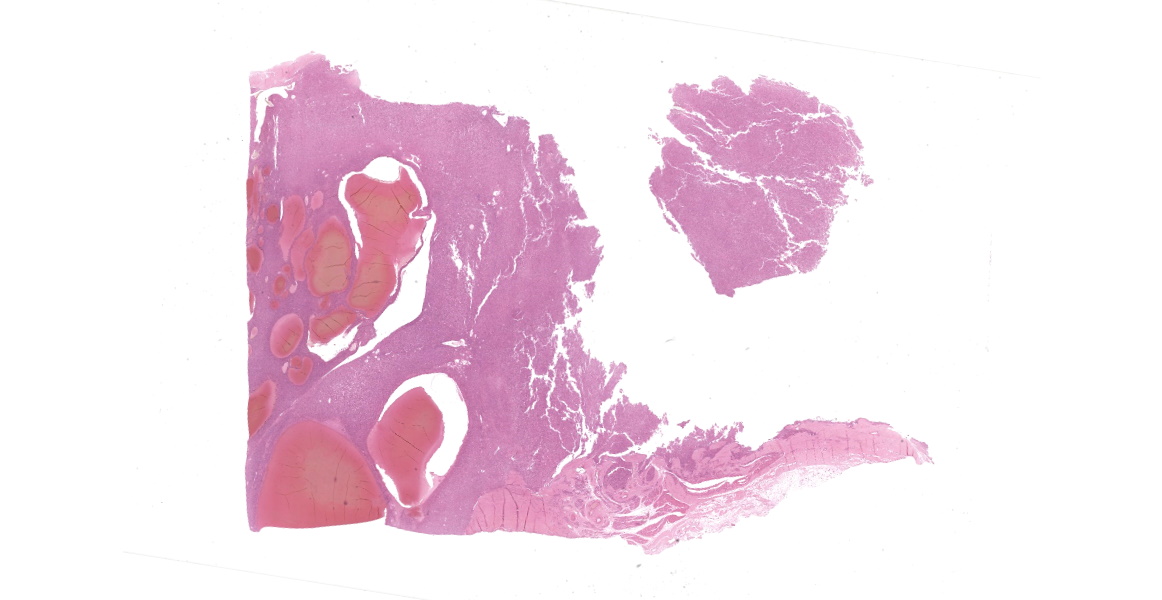
Left hind leg mass involving the bone and associated soft tissues (skin/muscle/subcutis).
Microscopic Description:
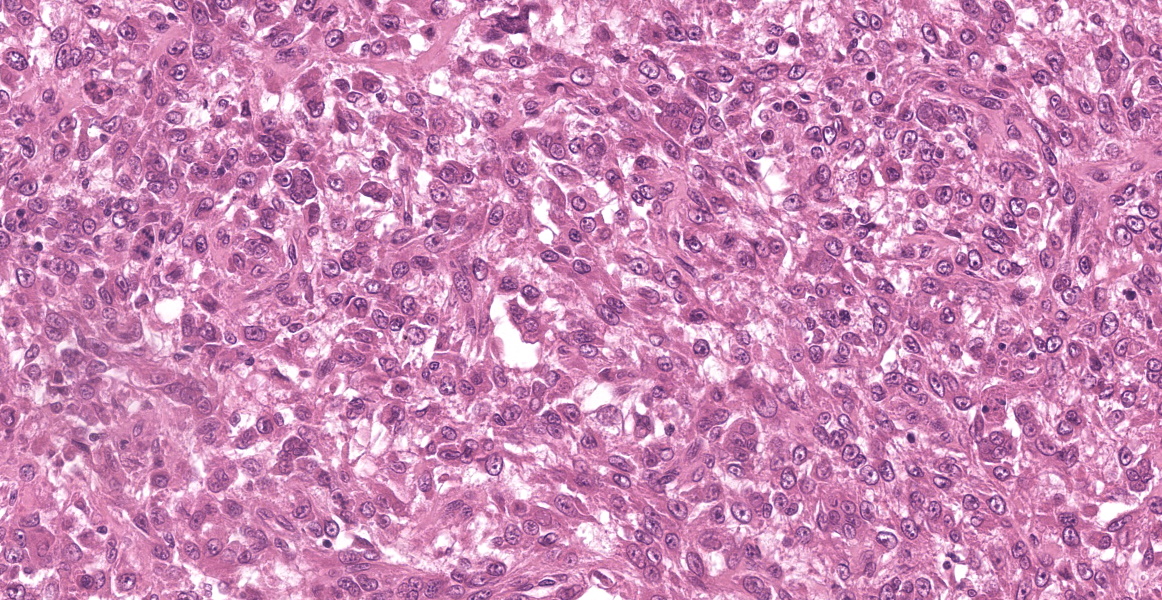
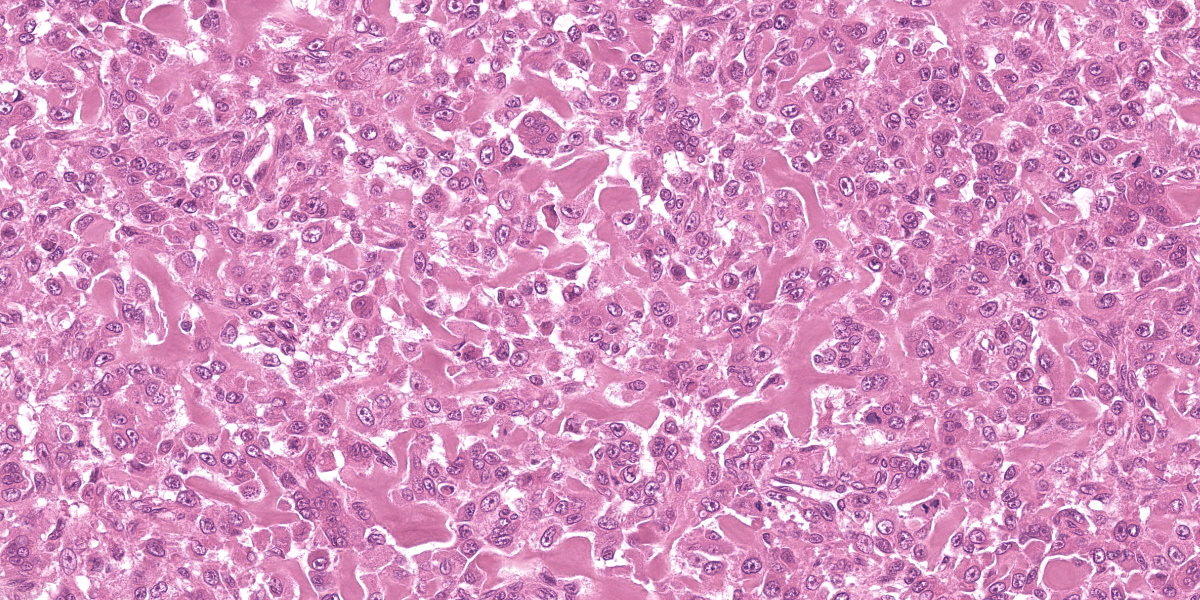
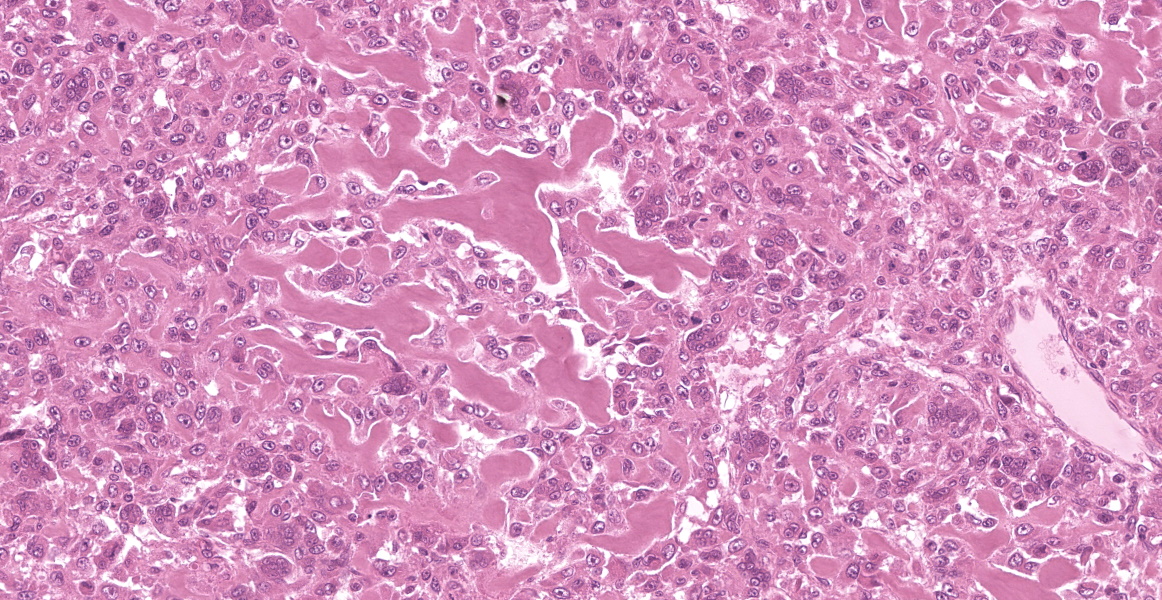
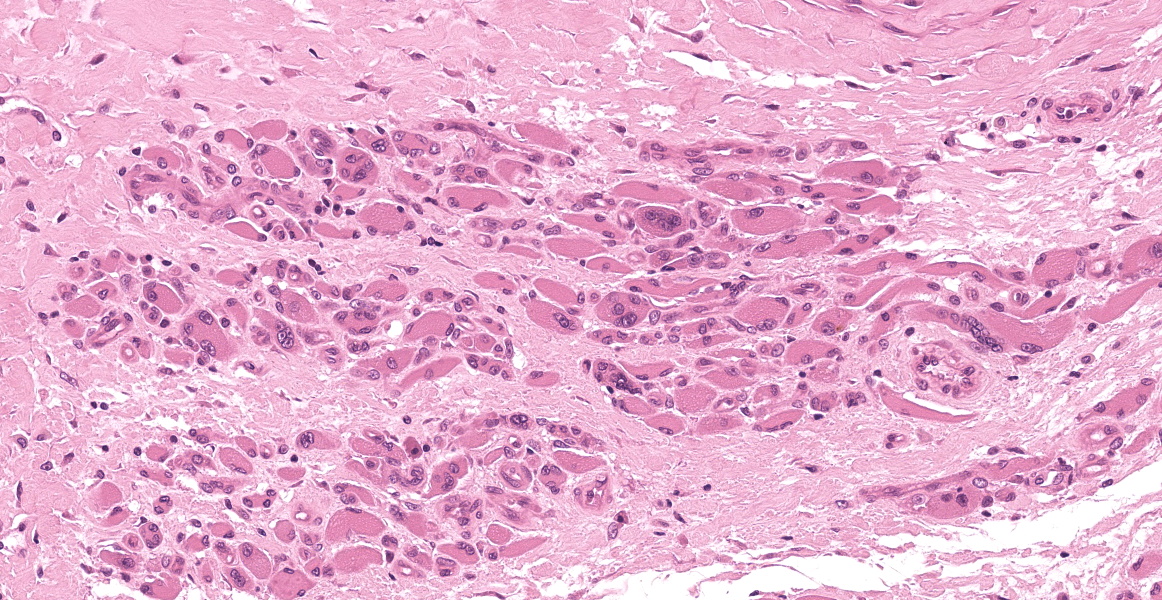
Multiple tissues from the amputated left hind leg received were examined. Sections of bone were examined after decalcification. The examined tissues contained a non-demarcated, non-encapsulated neoplasm composed of variably loose to solid sheets of spindle to polygonal cells supported on ample fibrovascular stroma. The cells had indistinct to variably distinct cell borders, ample eosinophilic cytoplasm, round to oval vesicular nuclei and distinct nucleoli. Six mitotic cells were observed in 2.4 mm2/equivalent to 10 N22/40x high power fields. Anisocytosis and anisokaryosis were moderate and there were multifocal multinucleated giant cells consistent with osteoclasts in the mass. The neoplastic cells multifocally surrounded eosinophilic fibrillar material (osteoid) and osseous trabeculae.
Contributor’s Morphologic Diagnosis:
Osteosarcoma, Metastatic.
Contributor’s Comment:
Osteosarcoma (OSA) is the most common primary bone tumor in dogs. In general, the risk of developing osteosarcoma appears to be amplified under conditions that drive excess osteoblast activation. OSA most commonly develops at or near growth plates, where cell turnover is highest, and represents up to 85% of all primary malignant bone tumors in the dog.4,5,7 Appendicular OSA is diagnosed most frequently in middle-aged to older, large and giant breed dogs, and affects the forelimb, particularly the humerus, more frequently than the hindlimb.
Significant differences in distribution patterns are reported between age, tumor location, and phylogenetic clusters. There are also significant variations in distribution patterns between neuter status, age, and dog sizes.7 High risk of appendicular osteosarcoma in large and giant breed dogs may often be the result of replicative mutations caused by normal processes of cell division required to create longer bones, with only modest contributions from heritable or environmental factors.4
Some studies identify breed associations with osteosarcoma risk in terms of both predisposition and protection. These results can inform breed health reforms, especially in breeds such as the Rottweiler, Rhodesian Ridgeback and the Great Dane which have been shown to be highly at risk.3 The skin or subcutaneous tissue is often the first osteosarcoma metastatic site detected as was observed in this case. After cutaneous and subcutaneous metastasis, the prognosis is grave with a median survival time of less than 2 months.5 Treatment with surgery and chemotherapy is suggested to improve outcome and was reported to be significantly associated with survival time after the diagnosis. The median cutaneous/subcutaneous metastasis-survival time for dogs treated with surgery and chemotherapy or chemotherapy alone was significantly longer than in the untreated ones, although the prognosis for dogs with metastatic tumors much poorer than for individuals with only primary tumors.6
Contributing Institution:
Tifton Veterinary Diagnostic and Investigational Laboratory
College of Veterinary Medicine
Department of Veterinary Pathology
University of Georgia.
https://vet.uga.edu/diagnostic-service-labs/veterinary-diagnostic-laboratory/.
JPC Diagnosis:
Skeletal muscle and tendon: Osteosarcoma.
JPC Comment:
Osteosarcoma is well-known and well-studied in veterinary medicine; however, despite the familiarity of this tumor, it remains difficult to differentiate OSA from other primary bone neoplasms, which often present with similar clinical signs and radiographic appearances.1 The issue is clinically urgent as OSA has a high risk of metastasis and an aggressive clinical course, yet histopathologic diagnosis of bone neoplasms was found to be only 72% accurate in one study, with only 13 of 18 malignant tumors accurately diagnosed.1 Of the misdiagnosed tumors, 3 were initially diagnosed as less aggressive tumors, but were ultimately proved to be OSA.1
Histologic differentiation of bone tumors is difficult primarily because of their morphologic similarities and the small tissue samples typically provided for examination. Because of this, various immunohistochemical (IHC) markers have been evaluated for their potential to increase the sensitivity and specificity of an OSA diagnosis; however, a single sensitive and specific marker to differentiate ostoblastic cells in formalin-fixed tissue has yet to be identified.1 A recent study in Veterinary Pathology characterized the expression patterns of four osteoblast-associated markers—Alkaline phosphatase (ALP), runx2, osteonectin, and osteopontin—to determine if the expression patterns of these proteins would be useful in differentiating OSA from other bone tumors.1
ALP is already used for this purpose in cytology, where staining for ALP enzyme activity on cytologic samples can increase the sensitity and specificity of an OSA diagnosis to 100% and 87%, respectively.1 In contrast, ALP IHC staining identifies the protein rather than its enzymatic activity, and, on formalin-fixed tissue, has an excellent sensitivity (100%) but only a limited specificity (30%), making it unreliable for diagnosing OSA as a sole marker.1 Runx2 is a transcription factor that is essential for osteogenesis, skeletal development, and osteoblastic differentiation. Its expression is consistently elevated in both human and canine OSA, and runx2 IHC staining was found to be 87% sensitive and 78% specific in the diagnosis of canine OSA.1
Based on the sensitivity and specificity profiles of the four examined markers, the authors found the greatest diagnostic benefit from running ALP and runx2 in series.1 The diagnostic algorithm requires running the ALP IHC first. Due to the high sensitity of ALP, if the sample is negative, the tumor is likely not an OSA and no further testing is necessary. If the tumor expressed ALP, however, runx2 should be interrogated to further characterize the tumor. When performed in series in this order, ALP and runx2 have a combined sensitivity of 87% and a specificity of 85% providing a useful set of diagnostic OSA markers.1
The moderator for this half of the conference, Dr. Meuten, used this classic entity to compare and contrast the traditional narrative, descriptive reports produced by most veterinary pathology diagnostic institutions, including this one, with the more streamlined, data point-driven process of synoptic reporting. After noting that synoptic reporting has become the standard in human pathology practice, Dr. Meuten led a robust discussion centered on the practical challenges associated with and the institutional and individual resistance to the transition.
Synoptic reporting dispenses with narrative descriptions and pares down diagnostic deliverables to discrete pieces of data reported in defined formats in a checklist-style report.2 As extensively discussed in conference, the challenge with synoptic reporting is choosing what data gets collected, what data gets reported, and to whom the data is disseminated. While it is facile to state that only data which has proven diagnostic, prognostic, and predictive value should be reported, in practice, this becomes complicated by practical considerations related to the need for future data mining, the different needs of different audiences, and what is considered diagnostic or prognostic enough to merit inclusion in the report.
In the context of this particular tumor, discussion focused on whether histologic subtype, which does not currently have clear predictive value, should be included on a hypothetical synoptic report template for OSA. Some residents thought that the examined OSA might be a telangiectatic subtype and felt that this information should be collected. The moderator countered that the potential oncologist reading the report would likely treat the animal the same, regardless of histologic subtype, so should the synoptic report include only the diagnosis?
The interesting, rhetorical discussion raged on for some time with the moderator passionately exploring the concept of synoptic reporting to an engaged but admittedly skeptical audience. An excellent discussion of the considerations involved in synoptic reporting can be found at the Veterinary Cancer Guidelines and Protocols website.2
References:
- Barger A, Baker K, Driskell E, et al. The use of alkaline phosphatase and runx2 to distinguish osteosarcoma from other common malignant primary bone tumors in dogs. Vet Pathol. 2022;59(3):427-432.
- Dark MJ, Bertram CA, Donovan TA, Meuten DJ, Miller AD, Moore FM. Synoptic Reporting Guideline, version 1.0. Veterinary Cancer Guidelines and Protocols. 2021. Available at: http://vetcancer protocols.org. Accessed on 15 April 2024.
- Edmunds GL, Smalley MJ, Beck S, et al. Dog breeds and body conformations with predisposition to osteosarcoma in the UK: a case-control study. Canine Med Genet. 2021;8(1):2.
- Makielski KM, Mills LJ, Sarver AL, et al. Risk factors for development of canine and human osteosarcoma: a comparative review. Vet Sci. 2019;6(2):48.
- Parachini-Winter C, Curran KM, Pellin M, et al. Cutaneous and subcutaneous metastasis of appendicular osteosarcoma in dogs: 20 cases. J Vet Intern Med. 2019;33:2200-2208.
- Simpson S, Dunning MD, Simone de Brot, et al. Comparative review of human and canine osteosarcoma: morphology, epidemiology, prognosis, treatment and genetics. Acta Vet Scand. 2017;59(1):71.
- Tuohy JL, Shaevitz MH, Garrett LD, Ruple A, Selmic LE. Demographic characteristics, site and phylogenetic distribution of dogs with appendicular osteosarcoma: 744 dogs (2000-2015). PLoS One. 2019;14 (12):e0223243.