Signalment:
Gross Description:
There are two sharp lines of demarcation, one that is noted at the beginning of the duodenum near the termination of the pylorus, and the other line is noted near the beginning of the jejunum. That area, which encompasses the duodenum, measures 15 cm in length, is diffusely dark red to black, and on cut surface, there is diffuse serial and mucosal hemorrhage. The jejunum, ileum, cecum, and colon contain multifocal dark red hemorrhagic areas on the serosa that measure 1.0 cm to 2.0 cm in diameter and contain a moderate amount of hemorrhagic luminal content.
Histopathologic Description:
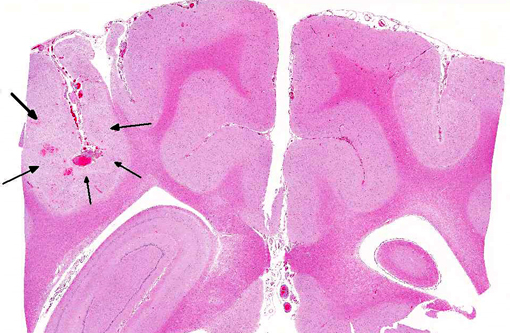
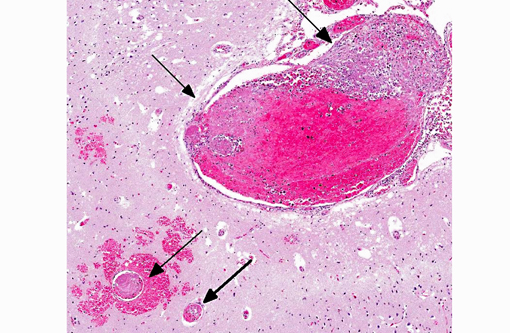
Additional findings (not included in slide set): The same neoplastic population of cells was seen within vascular lumens in the following organs: cervical and thoracic spinal cord, left and right bulla, liver, spleen, lung, lymph node, adrenal glands, and duodenum. Other histological findings in this animal included: moderate lymphoplasmacytic and neutrophilic cellular infiltrate within the epithelium of the middle ear of the left bulla; diffuse submucosal hemorrhage in the right bulla; diffuse hemorrhagic pancreatic necrosis; transmural duodenal hemorrhage, and multifocal mucosal hemorrhage in the remainder of the intestinal tract.
Morphologic Diagnosis:
Lab Results:
Blood smear: unremarkable.
The neurological exam findings included the following: depressed, dull mental status; right sided head tilt and circling to the right; mild amount of pain elicited on palpation of cranial cervical region; no vestibulo-ocular response present; positional ventral strabismus OD; and anisocoria (L>R). Lesion localization was central vestibular disease (vestibular signs with altered mentation).
Tick panel, 4 DX (Ehrlichia, Anaplasma, Heartworm, Lyme), and Cryptococcus antibody: all negative.
MRI: multifocal regions of parenchymal T2 hyperintensity and increased meningeal enhancement with evidence of parenchymal hemorrhage consistent with a severe meningoencephalitis, and focal regions of compensatory hydrocephalus consistent with prior parenchymal necrosis, and left otitis media.
CSF analysis: mononuclear pleocytosis (NCC19). Otic cytology: septic suppurative inflammation. Otic culture: Staphylococcus pseudintermedius.
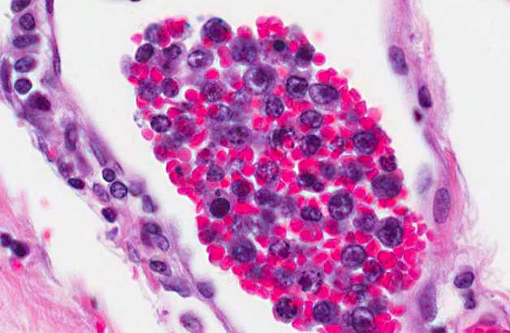
CD3 and CD79a immunohistochemical stains were applied to the tissue sections of cerebrum. The positive controls worked well and internal control lymphocytes were positive. CD3 immunostain revealed minimal to mild, occasional membrane staining of individual neoplastic cells within vessels. The CD79a immunostain was negative.
PARR (PCR for antigen receptor rearrangement) results for cerebrum: TCRγ PARR produced a crisp band in duplicate of the appropriate size that persisted upon heteroduplex analysis. BCR PARR was negative. PARR interpretation: These results should raise your suspicion of T cell neoplasia.
Condition:
Contributor Comment:
Although this neoplasm is rare, the disease in dogs has been reported to have a predilection for CNS, lung, and less commonly skin.(3,4) One retrospective review of cases of canine IVL found that the most common clinical presentation in dogs included spinal cord ataxia, seizures, vestibular disease, and posterior paralysis.(4) The laboratory findings (CBC, chemistry panel) in the dogs in this study, as well as the dog in this case, were non-specific. Intravascular lymphoma appeared to affect mostly large breed dogs with an average age of six years.(4) The diagnosis of IVL is predominantly a postmortem diagnosis; however, there is one report of an antemortem diagnosis achieved by CT-guided biopsy of a brain lesion.(2) Intravascular lymphoma is typically rapidly progressive; death is reported to occur from 20 days to six months after the first reported clinical signs.(2)
Although the reason for the tendency of neoplastic cells to remain within vessels has not been definitively proven in dogs, several human studies have demonstrated an absence of leukocyte adhesion molecules such as CD11a/CD185,7 and CD29,6 in neoplastic cells. Based on the findings, it was hypothesized that due to the lack of adhesion molecules, the neoplastic cells could not extravasate.
JPC Diagnosis:
1. Cerebrum: Intravascular lymphoma.
2. Midbrain, gray matter and meninges: Vascular necrosis and thrombosis, multifocal, severe with infarction.
Conference Comment:
Integrins mediate stable adhesion, which must be preceded by activation, margination and rolling. Leukocytes that normally express integrins in low affinity state are activated by chemokines, while L-selectins are cleaved from the neutrophil surface by A Disintegrin and Metalloproteinase 17 (ADAM17). This results in the conversion of β1integrins, such as VLA-4 (α4β1, CD49d/CD29), and β 2integrins, such as LFA-1 (CD11a/CD18), Mac-1 (CD11b/CD18, CR3), gp150, 95 (CD11c/CD18, CR4) and αdβ2 (CD11d/CD18) to a high affinity state. Inflammatory mediators also induce endothelial expression of ligands for β1/β2-integrins. These ligands generally belong to the immunoglobulin superfamily. Most β2-integrins on appropriately stimulated leukocytes firmly adhere to ICAM-1 (CD-54) on endothelial cell; αdβ2, on the other hand, binds ICAM-3. Alternatively, β 1integrins on lymphocytes, monocytes and high endothelial venules (HEVs) bind VCAM-1 on endothelial cells (Table 1).(1)
During transmigration, leukocytes emigrate between endothelial cells, primarily at intercellular junctions in post-capillary venues. This is mediated by adhesion molecules, which vary slightly depending on the leukocyte and tissue type. PECAM-1 (CD31) and CD99 on leukocytes bind homotypically to PECAM-1 and CD99, respectively on endothelial cells. Additionally, leukocyte junctional adhesion molecules JAM-A and JAM-B can bind to JAMA and JAM-C at endothelial cell junctions. Leukocyte integrins also play a role in transmigration. Leukocytes extend pseudopodia between endothelial cells to interact with basement membrane laminins and collagen as well as extracellular matrix proteins such as proteoglycans, fibronectin and vitronectin. Leukocyte binding to these proteins, primarily via β1-integrins, enables leukocytes to transmigrate into perivascular tissue, from which they can migrate along chemotactic gradients toward the area of injury. Whether endogenous (e.g., cytokines, C5a, arachidonic acid metabolites) or exogenous (e.g., bacterial) substances, chemotactic agents bind to specific G-protein coupled receptors and activate second messengers, which induces actin polymerization and allows filopodia to pull the cell into the area of inflammation. In connective tissue, leukocytes can adhere to the extracellular matrix and advance via β1-integrins (Table 2).(1)
Deficiencies in β1 (CD29) or β2-integrins (CD18) have been reported in the neoplastic cells in human intravascular lymphoma. Although other defects are likely involved as well, this lack of leukocyte adhesion molecules may render neoplastic lymphocytes unable to successfully exit the blood vessel, resulting in the intravascular cellular accumulation characteristically noted in this neoplasm. A similar leukocyte adhesion deficiency has not yet been demonstrated in canine intravascular lymphoma, and its molecular basis remains unknown.(4,5,6)
Table 1: Select endothelial cell and leukocyte adhesion molecules1
| Endothelial Molecule | Leukocyte Receptor | Major Role |
| P-selectin | PSGL-1 (a Sialyl-Lewis-X glycoprotein) | Rolling (neutrophils,monocytes, lymphocytes) |
| E-selectin | ESL-1 (a Sialyl-Lewis-X glycoprotein); Sialyl-Lewis A glycoprotein | Slow rolling, adhesion to active endothelium (neutrophils, monocytes, Tcells) Important in homing of effector & memory T-cells, especially to skin |
| ICAM-1 | β2-integrins: LFA-1 (CD11a/CD18), Mac-1 (CR3; CD11b/CD18); gp150,95 (CR4; CD11c/CD18) | Adhesion, stable adhesion, transmigration (all leukocytes) |
| VCAM-1 | β1-integrins: VLA-4 (α4β1; CD49d/CD29), LPAM-1 (α4β7) | Adhesion (eosinophils, monocytes, lymphocytes) VLA-4 mediates homing of lymphocytes to endothelium at peripheral sites of inflammation |
| GlyCAM-1; MadCAM-1; CD34 | L-selectin | Rolling; lymphocyte homing to high endothelial venules (HEV) Also serves to bind neutrophils to activated endothelium |
| PECAM (CD31) | PECAM (CD31) | Transmigration; Leukocyte migration through endothelium |
| CD99 | CD99 | Transmigration |
| JAM-A | JAM-A, LFA-1 | Transmigration |
| JAM-C | JAM-B, Mac-1 | Transmigration |
Table 2: Select leukocyte integrins and extracellular matrix components involved in leukocyte chemotaxis1
| Leukocyte Integrin | ECM Component |
| VLA-1, 2 | Collagen |
| VLA-3, 5 | Fibronectin |
| VLA-6 | Laminin |
References:
2. Bush WW, Throop JL, McManus PM, et al. Intravascular lymphoma involving the central and peripheral nervous systems in a dog. J Am Anim Hosp Assoc. 2003;39(1):90-96.
3. Ginn PE, Mansell JEKL, Rakich PM. Skin and appendages. In: Maxie, MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 1. Philadelphia, PA: Elsevier; 2007:775.
4. McDonough SP, Van Winkle TJ, Valentine BA, et al . Clinicopathological and immunophenotypical features of canine intravascular lymphoma (Malignant Angioendotheliomastosis). J Comp Pathol. 2002;126(4):277-288.
5. Ossege LM, Postler E, Pleger B, et al. Neoplastic cells in the cerebrospinal fluid in intravascular lymphomatosis. J Neurol. 2000;48 (8):656-658.
6. Ponzoni M, Ferreri AJM. Intravascular lymphoma: a neoplasm of homeless lymphocytes? Hematol Oncol. 2006;24(3): 105-112.
7. Valli VEO. Hematopoietic system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 3. Philadelphia, PA: Elsevier;2007:183.