WSC 22-23
Conference 14
Case III:
Signalment:
11 month old male neutered Rottweiler, Canis lupus familiaris, canid/domestic dog
History:
An 11-month-old neutered male Rottweiler dog was euthanized 21-days after routine castration under general anesthesia for severe generalized polymyopathy. The dog was reportedly clinically normal prior to the procedure. Ten days following anesthesia the dog presented recumbent, with severe generalized muscle weakness, azotemia, myoglobinuria, and a markedly increased serum creatinine kinase (CK) concentration of 806,080 U/L. With supportive care over the next two weeks the dog’s strength and coordination returned, serum CK decreased to 980 U/L, azotemia improved, and myoglobinuria resolved. Twenty-one-days post anesthesia/surgery, the dog again became acutely weak and lethargic and was humanely euthanized.
Gross Pathology:
Submitted is the body of a 30 kg brown and black male neutered Rottweiler with adequate fat stores and diffuse decreased symmetrical muscle mass most prominent of the epaxial and gluteal muscles. Mucous membranes are pale pink to white and tacky. There is mild tan/brown tartar on all canines, premolars, and molars. The ventral abdomen from the xiphoid process to the pubis, both antebrachia, the medial aspect of the left and right metatarsus, the lateral aspect of the left and right tarsus, and the scrotum have been shaved. There is yellow vetwrap wrapped circumferentially around the right tarsus. Autolysis is moderate.
External examination:
Paw pad, right forelimb: There is a 3.5 x 0.5-1.0 cm, slightly depressed, well-demarcated, loss of the epidermis with jagged margins that extend the width of the right metacarpal paw pad. The center is mottled tan and pink. There is a similar circular, 1 cm diameter, lesion of the right carpal paw pad. The center is red to brown with serous crusting.
Skin around neck: On either side of the neck there are 2 skin sores that are 4 x 1.5 cm (right-side), and 3.5 x 1 cm (left-side). Both sores are well-demarcated with loss of haired skin and a slightly depressed, crusty, pink center. Within the shaved patch of hair on the left antebrachium there is a similar 2.5 x 2.5 cm sore. Scrotum: The skin of the scrotum is light pink. The scrotal incision is intact with little reaction.
Internal Examination:
Skeletal muscle: Throughout the entire body the skeletal muscles are subjectively soft and diffusely pale pink with thin to broad streaks of tan. Most severely affected muscles are within the hindlimbs, epaxial, and seratus muscles, with ~50% of these muscle bundles affected. There are a few small thin streaks of tan in bilateral temporalis muscles, affecting <5% of the tissue. There are a few (~5) tan foci (up to 1 cm in diameter) in bilateral masseter muscles, affecting <5% of the tissue. The diaphragm is diffusely pale and subjectively thickened up to 3 mm.
Heart: In the endocardium of the left atrium there are 4 adjacent raised exophytic nodules. Three of these nodules are small (up to 3 mm), tan to white, firm, with a smooth outer surface. The fourth nodule is larger, ovoid shaped, 8 x 5 mm, 4 mm raised, and red with speckles of tan. It is solid and red on cut section. Surrounding these nodules are multiple (~10) linear to focal, 1-3 mm, hard, raised plaques (suspect jet lesions). Just superior to the pulmonic valve and the aortic valve, there are approximately 2 x 1 cm area that contains multiple previously described hard plaques (suspect jet lesions). The free margins of the right and left atrioventricular valve leaflets are short, white, firm, and mildly thickened. The valve surface remains smooth and glistening.
Kidneys: The kidneys are mottled red/brown to green (suspect autolysis). The cortical surface of both kidneys contains multiple (~50 per kidney) up to 3 mm diameter depressions. On cut section the cortex contains alternating radiating streaks of red/brown and tan.
Spleen: In the capsule at the edge of the head of the spleen there is a dark purple to black 1.0 x 1.0 cm, well demarcated, irregular shaped, depression that is outlined by a 2 mm periphery of tan. This extends slightly into the parenchyma as a tan foci. At the head of the spleen, near the edge, there is also a 0.7 cm x 0.4 cm silver, shiny, plaque adhered to the capsule that does not extend into the parenchyma. Throughout the splenic parenchyma there are hundreds of white foci (0.5 mm to 2 mm in diameter) (suspect lymphoid follicles).
Lungs: The caudal lung lobes are heavy and exude a moderate amount of red-tinged fluid. Subcutaneous tissue: There is a moderate amount of thin light yellow fluid within the subcutaneous tissues surrounding the hindlimbs.
Laboratory Results:
Clinical Pathology:
Day of discharge
- Urea 10.6 H mmol/L 3.5-9.0
- ALT 317 H U/L 19-107
- CK 980 H U/L 40-255
- Amylase 1617 H U/L 299-947
Day of readmission before euthanasia
- Urea: 27.5 mmol/L
- ALT 739 U/L
- CK 806,080 U/L
- Amylase 1810 U/L
Comparative Neuromuscular Laboratory report: Cryosection from the epaxial muscle and an archived control muscle were incubated with several polyclonal and monoclonal antibodies against dystrophy associated proteins including the rod and carboxy-terminus of dystrophin, utrophin, spectrin, laminin alpha2 (4F11), dysferlin (hamlet), alpha, beta and delta -sarcoglycans, beta-dystroglycan, caveolin 3, developmental myosin heavy chain (dMHC), MHC-I, collagen VI, emerin, the T cell markers CD3, CD4, and CD8, the B cell marker CD21 and the macrophage/dendritic cell marker CD11c. The staining intensity for rod-domain of dystrophin was normal in appearance. Moderately reduced staining intensity was present with the antibody against the carboxy-terminus of dystrophin and dystrophin associated proteins. Regenerating fibers were highlighted by dMHC staining. Utrophin staining was not increased. Antibody staining for laminin alpha2, dysferlin, caveolin3, collagen VI and emerin were normal in appearance. Several MHC-I and CD11c positive cells were present around necrotic fibers. Sporadic T cells (CD3+, CD4+ & CD8+) were observed. No CD21+ B cells were present. Spectrin2 staining was very weak suggesting partial tissue degradation."
- Comment: This may be dystrophin deficient muscular dystrophy with normal staining for rod domain and weak staining for the carboxy terminus. … While I still favor a diagnosis of dystrophin deficient MD, and episode of severe rhabdomyolysis should be ruled out by the clinical history and progression."
Microscopic Description:
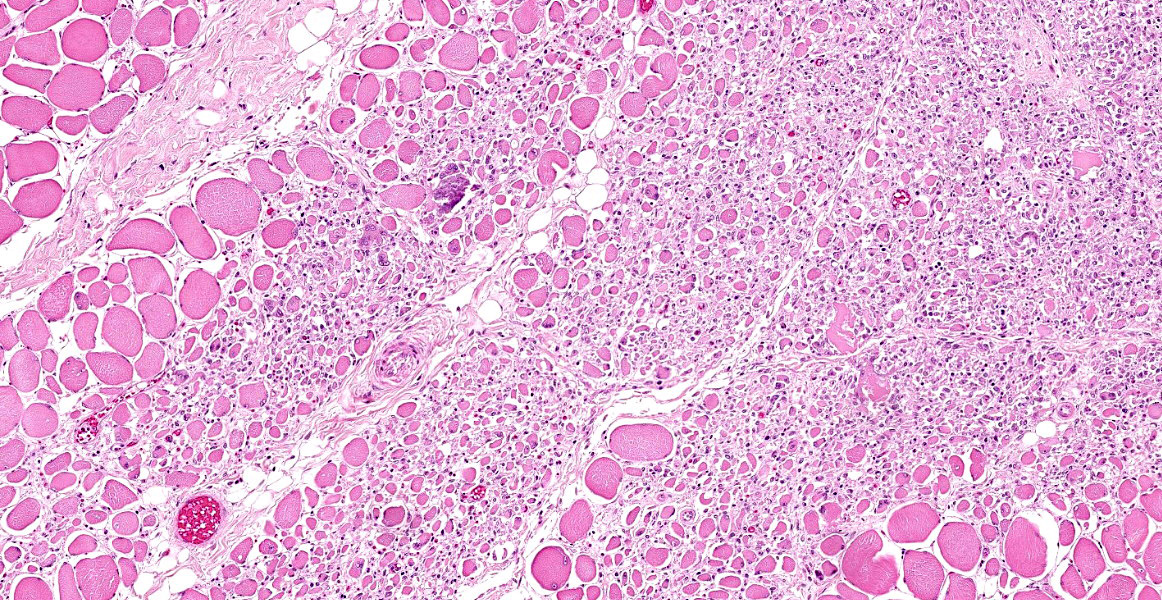
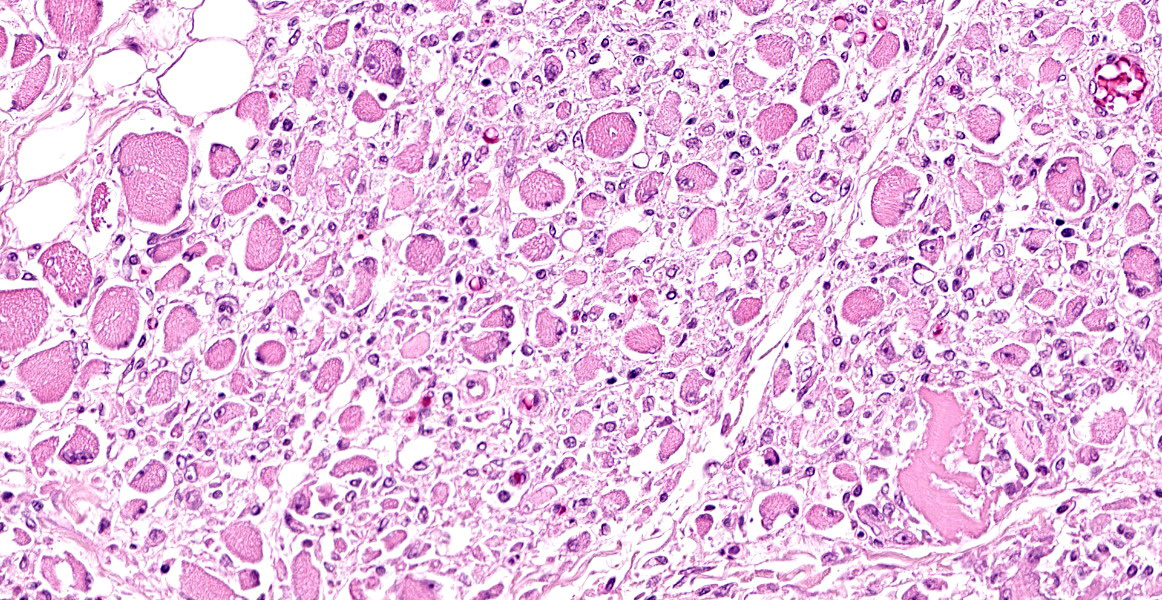
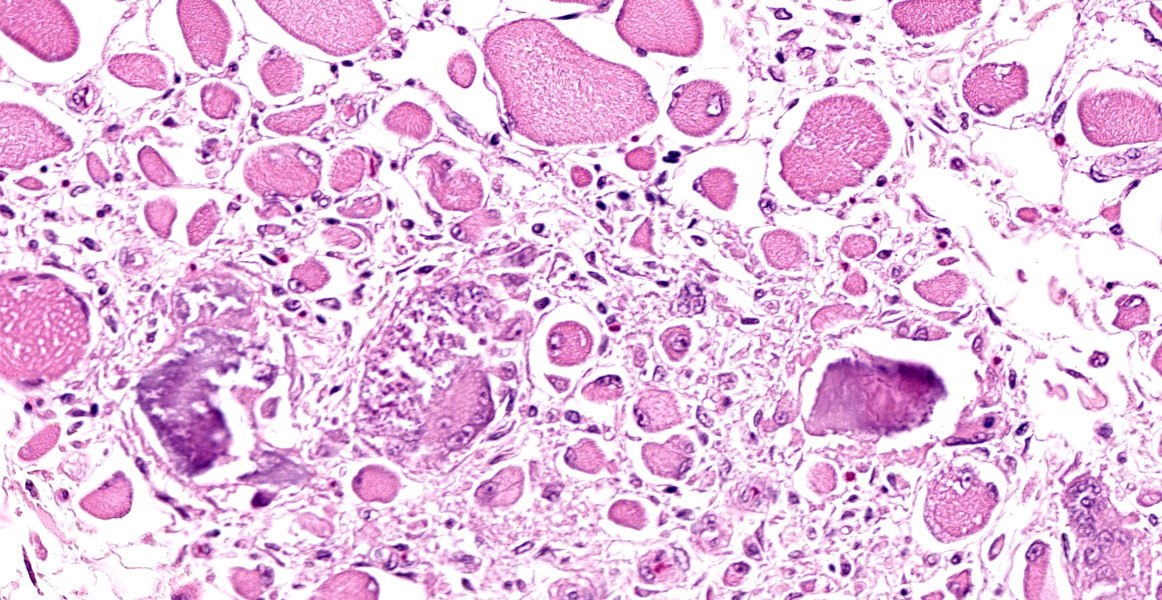
Skeletal muscle: All examined skeletal muscles have similar histologic features but vary in severity with up to 50% of myofibers affected. There are large groups of degenerating fibers at different stages of necrosis characterized by: single swollen hypereosinophilic fibers (hypercontraction) with loss of cross-striations, vacuolated cytoplasm and groups of thin, angular fibers with large plump peripheral nuclei. In addition, the cytoplasm of multiple randomly distributed swollen myofibers is replaced by basophilic granular to crystalline matrix (mineral confirmed by Von kossa stain) affecting up to 20% of the affected myofibers in one section (left semimembranosus muscle was most severe). Occasionally there are aggregates of foamy macrophages replacing single myofibers and scattered within the endomysium. Within the large groups of degenerating myofibers there are also single degenerating myofibers characterized by small rounded myofibers with multiple central nuclei (multinucleate muscle giant cells).
Contributor's Morphologic Diagnoses:
Skeletal muscle (generalized): Myonecrosis, degeneration and regeneration, multifocal polyphasic, subacute, severe.
Contributor's Comment:
Differential diagnoses for non-inflammatory canine polymyopathies include, amongst others, congenital or inherited myopathies, rhabdomyolysis, and malignant hyperthermia. Due to the young age of this dog, its breed (Rottweiler), and the gross and histologic findings, an inherited myopathy such as muscular dystrophy was initially suspected.
The most prevalent type of muscular dystrophies are the X-linked dystrophies. Of these, Duchenne’s muscular dystrophy (DMD) is the most common followed by Becker muscular dystrophy (BMD. Both dystrophies are caused by a recessive mutation of the large dystrophin gene on the short branch of the X-chromosome encoding the dystrophin protein. Dystrophin is an important protein in stabilization of the myofiber during contraction. Complete loss of dystrophin, as in DMD, or partial loss of dystrophin, as in BMD, makes myofibers particularly susceptible to damage. Damaged myofibers leak the muscle-specific enzyme CK which correlates with the often chronically elevated serum CK seen in these cases. Both myofiber necrosis and regeneration within the same muscle is characteristic of the histologic appearance, as a result of repetitive damage.
Canine X-linked muscular dystrophy, specifically DMD, was first described in the Golden retriever in the early 1980s and has since been reported in multiple dog breeds including the Rottweiler. One case of muscular dystrophy resembling BMD in humans was described in a Labrador retriever. In addition, a unique form of muscular dystrophy exists in Rottweilers known as juvenile-onset distal myopathy. This condition has been identified in young dogs with signs of muscle weakness since birth and characterized by skeletal muscle lesions of predominantly the distal limbs. Two dogs in this study had normal immunoreactivity for the dystrophin protein.
Juvenile-onset distal myopathy likely has a different pathogenesis from DMD and a metabolic defect may play a role. In addition, an inherited non-dystrophin myopathy, known as X-linked myotubular myopathy has also been reported in young Rottweilers, caused by a missense mutation in the MTM1 gene. This inherited condition is characterized by myofibers with central nuclei. Myonecrosis is not a feature.
In this case a diagnosis of muscular dystrophy is supported by the dog’s age, breed, gross and histologic findings. However, a defect of the dog’s dystrophin protein was not identified by immunohistochemistry and the dog’s clinical history is not consistent with past reports of canine muscular dystrophy. Prior to anesthesia/surgery, this dog was reportedly clinically normal with no evidence of muscle weakness.
In addition to muscular dystrophies, a diagnosis of rhabdomyolysis was also considered. Rhabdomyolysis is a condition of acute skeletal muscle necrosis. In dogs it is often associated with painful muscles, limb weakness, markedly increased CK, and myoglobinuria.
Rhabdomyolysis is often attributed to over-exertion or trauma but has also been associated with exposure to anesthetic agents, toxin exposure, and drug reaction/overdoses, amongst other things. In this case, a single episode of severe rhabdomyolysis after exposure to inhalant anesthesia is consistent with the myoglobinuria, acute kidney injury, and improved CK. However, rhabdomyolysis does not fit with the histologic findings of multifocal polyphasic myonecrosis and regeneration as rhabdomyolysis is typically characterized by a monophasic pattern of necrosis.
An episode of malignant hyperthermia was also considered. Malignant hyperthermia is an inherited disorder of skeletal muscle characterized by hypermetabolism and contracture and often occurs after exposure to anesthetic agents, exercise, or stress. In humans, pyrexia, hyperkalemia, hypercapnia, systemic acidosis, muscle rigidity, elevated CK levels, and often cardiac arrest are seen. Malignant hyperthermia, occurs sporadically in dogs with similar clinical findings. As in humans, canine malignant hyperthermia develops as a result of a mutation of the RYR1 gene, causing uncontrolled calcium-release by skeletal muscles, excessive contraction, and subsequent heat production. Without immediate post-anesthetic bloodwork to support an episode of malignant hyperthermia, evidence of peri-anesthetic pyrexia or cardiac abnormalities, it is difficult to make this diagnosis.
Contributing Institution:
Department of Pathobiology, Ontario Veterinary College, University of Guelph https://ovc.uoguelph.ca/pathobiology/
JPC Diagnosis:
Skeletal muscle, myofibers: Degeneration, necrosis, regeneration and loss, polyphasic, multifocal, with mineralization.
JPC Comment:
The contributor provides good differentials for consideration in this case of polyphasic skeletal muscle necrosis. X-linked muscular dystrophy has been reported in the dog, cat, mouse, and pig.1,2 The disease, which manifests in adolescents, is characterized by progressive muscle atrophy and weakness, though in the cat, mouse, and rat terrier, marked muscular hypertrophy may be seen.2 Severely affected animals may die shortly after birth. Older animals may develop dyspnea due diaphragmatic fibrosis and contracture, esophageal dysfunction (and potential regurgitation), exercise intolerance, and a stiff gait. The disease also affects the cardiac muscle and in dogs progresses to degenerative cardiomyopathy. Macroglossia is an interesting feature documented in dogs, cats, humans, and, most recently, a pig. In a recent article, Aihara et al described macroglossia in a 6-month-old pig caused by Becker muscular dystrophy. The underlying genetic mutation was pseudoexon insertion in the dystrophin gene, and histologically, the skeletal muscle was replaced by abundant adipose and fibrosis, causing gross enlargement of the tongue (pseudohypertrophy).1
Cardiomyopathy is now the leading cause of death in young men with Duchenne’s muscular dystrophy (DMD).5 Gross and histologic lesions of the heart were described in a recent study of 26 dogs with golden retriever muscular dystrophy (GRMD), an animal model for DMD.5 Gross lesions, seen in dogs over 10 months of age, included myocardial pallor and streaking in both ventricles, papillary muscle fibrosis or mineralization, and dilation of one or both ventricles.5 Histologically, there was significant fatty infiltration and degeneration, most prominently within the sub-epicardium and also affecting papillary muscles, and lesion severity correlated positively with age.5 Affected dogs also had arteriolar medial hypertrophy, and 11 of 26 had aortic mineralization.5 Acute coagulative myocardial necrosis was seen in many affected dogs.5 There was no evidence of thrombosis in histologic sections, and semi-quantitative necrosis scores were correlated with the degree of arteriolar hypertrophy.5 These findings led the authors to suspect that the necrosis was caused by dysregulation of vascular tone causing functional ischemia coupled with increased susceptibility of dystrophin-deficient cardiomyocytes to hypoxia.5
The effects of DMD on vascular smooth muscle was the subject of another recent research article, which evaluated dystrophin expression, nitric oxide synthetase (NOS) activity, and endothelial nitric oxide synthetase (eNOS) expression in large arteries of 16 DMD dogs compared to 15 unaffected dogs.3 Unlike normal dogs, DMD dogs lacked dystrophin in smooth muscle and endothelial cells of the arteries and vena cava.2 Additionally, DMD had significantly lower endothelial NOS activity and eNOS expression compared to normal dogs.3 This is attributed to the fact that dystrophin is used to anchor NOS to the sarcolemma; in cells lacking dystrophin, NOS is delocalized and less effective.3 Affected arteries had both decreased vasoconstriction and decreased vasodilation in response to endogenous signals, and the arteries were also smaller with decreased wall thickness attributed to tunica media atrophy.3 This conflicts with the findings of Schneider et al, in which arteriolar hypertrophy was seen; however, the type of vessel analyzed differed between the two studies (cardiac arteriole versus femoral artery), and both studies agreed that vascular defects may play a role in DMD pathogenesis.3,5
References:
- Aihara N, Kuroki S, Inamuro R, et al. Macroglossia in a pig diagnosed as Becker muscular dystrophy due to dystrophin pseudoexon insertion derived from intron 26. Vet Pathol. 2022: 59(3): 455-458.
- Cooper BJ, Valentine BA. Muscle and Tendon. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 1. 6th St. Louis, MO: Elsevier, 2016. 192-196.
- Hanson SM, Smith MO, Walker TL, Shelton GD. Juvenile-onset distal myopathy in rottweiler dogs. J Vet Internal Med.1998; 12: 103.
- Kodipilli K, Thorne PK, Laughlin MH, Duan D. Dystrophin deficiency impairs vascular structure and function in the canine model of Duchenne muscular dystrophy. J Pathol. 2021; 254(5):589-605.
- Roberts MC, Mickelson JR, Patterson EE, Nelson TE, Armstrong PJ, Brunson DB, Hogan K. Autosomal dominant canine malignant hyperthermia is caused by a mutation in the gene encoding the skeletal muscle calcium release channel ( ryr1). Anesthesiology. 95: 716-725.
- Schneider SM, Snsom GT, Guo L, Furuya S, Weeks BR, Kornegay JN. Natural History of Histopathologic Changes in Cardiomyopathy of Golden Retriever Muscular Dystrophy. Front Vet Sci. 2022; 8:759585.
- Shelton GD. 2004. Rhabdomyolysis, myoglobinuria, and necrotizing myopathies. Vet Clin Small Anim. 2004; 34: 1469-1482.
- Shelton GD, Liu LA, Guo LT, Smith GK, Christiansen JS, Thomas WB, Smith MO, Kline KL, March PA, Flegel T, Engvall E. Muscular dystrophy in female dogs. J Vet Internal Med. 2001; 15: 240-244.
- Shelton GD, Rider BE, Child G, Tzannes S, Guo LT, Moghadaszadeh B, Troiano EC, Haase B, Wade CM, Beggs AH. X-linked myotubular myopathy in rottweiler dogs is caused by a missense mutation in exon 11 of the mtm1 gene. Skeletal Muscle. 2015; 5: 1