Signalment:
Gross Description:
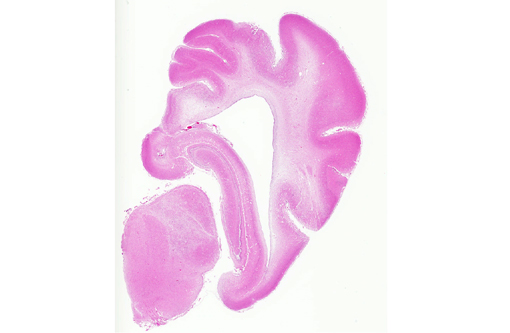
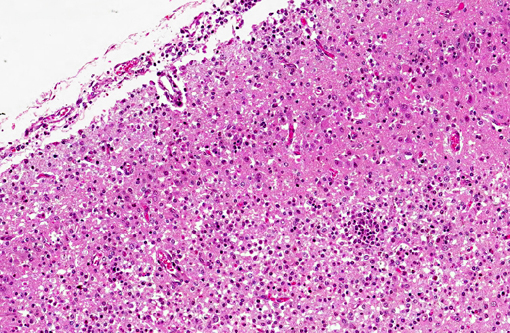
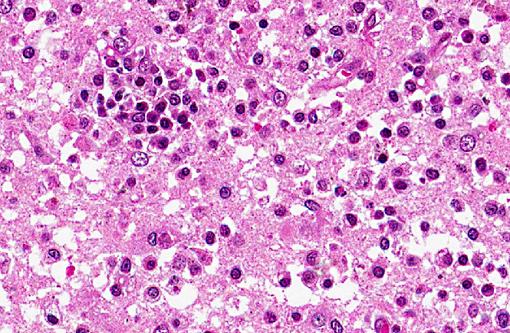
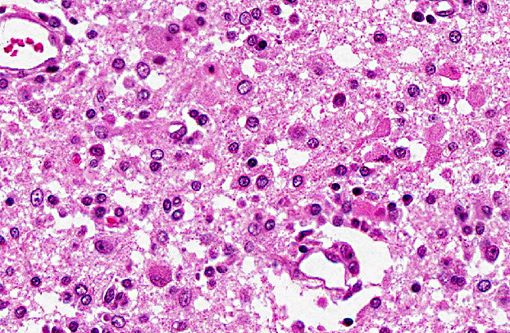
Histopathologic Description:
Spinal cord (not included): There was nonsuppurative poliomyelitis characterized by perivascular cuffing with mononuclear cells, diffuse and multifocal gliosis and severe neuronal necrosis. JEV antigen was immunohistochemically detected in the cytoplasm of nerve cells within the lesions.
Histological lesions and viral antigen were restricted to the central nervous system. There were no lesions in the other examined organs including the liver, spleen, kidney, heart, lung and lymph node.
Morphologic Diagnosis:
Lab Results:
- No significant bacteria or viruses were isolated from the fetus.
- JEV Hemagglutination inhibition (HI) titers from the body fluid of stillborn piglets: ~10 to ~640.
- JEV HI titers from sows that aborted: ~640 to ~5120.
- Serological tests for porcine reproductive respiratory syndrome virus (PRRSV), porcine parvovirus (PPV), ADV and toxoplasmosis were all negative.
- PCR for PRRSV and PPV were negative.
Condition:
Contributor Comment:
In the present case, JEV antigen was immunohistochemically detected in the cytoplasm of intralesional nerve cells. An immunohistochemical method for diagnosing JEV in formalin-fixed tissues from infected pigs would be useful as a simple and rapid diagnostic test. JEV antigen was immunohistochemically detected in the nerve cells within areas of nonsuppurative encephalitis in 3-week-old pigs experimentally infected with JEV.(12,13) However, postmortem changes prevent immunohistochemical detection of viral antigen in many cases of aborted fetuses. Therefore, there are limited reports of immunohistochemical detection of JEV antigen in aborted fetuses. The present case shows that immunohistochemical detection of JEV antigen is also of diagnostic importance in those aborted fetuses with a relative lack of postmortem change.
In the present case, the virus was not isolated from stillbirth fetus. It appears that successful isolation of JEV from pigs of abnormal litters is dependent on the time that pigs were exposed to the virus in utero. It was reported that JEV was successfully isolated from feti of 3 litters that were collected at 7 to 22 days after experimental inoculation of sows with JEV, but not from affected pigs of 2 litters that were collected 62 and 84 days after infection of sows.(11) Detection of JEV specific antibody in body fluids of aborted fetuses by HI is a useful method for diagnosis.(10) The present case was diagnosed as Japanese encephalitis based on clinical history, histopathological findings, detection of JEV specific antibody by HI and immunohistochemical detection of JEV antigen in the brain lesions.
JPC Diagnosis:
Conference Comment:
As its name implies, Japanese encephalitis virus primarily occurs in Asia and Southeast Asia, especially in areas with extensive freshwater marshes or irrigated rice fields. The primary vector is the Culex mosquito.(7) Swine do not typically develop clinical disease when infected with this flavivirus (with exceptions as noted by the contributor); they function as disease amplifiers and they are often used as sentinels for monitoring Japanese encephalitis virus in endemic areas. In addition to swine, wild birds have been implicated as amplifying hosts in virus transmission.(3) Viral titers in horses and humans are likely insufficient for re-infection of mosquito vectors, so these species are considered dead-end hosts.(3) Japanese encephalitis virus is one of the most frequent causes of human encephalitis in Asia(3) and it can also cause fatal encephalitis in horses.(12) Routes of flaviviral neuroinvasion remain somewhat controversial. Historically, hematogenous spread to the CNS was the presumed route of infection, however recent studies also support retrograde axonal transport via olfactory nerves.(2,13)
West Nile virus is also transmitted by Culex mosquitoes, with wild birds functioning as the primary amplifying hosts.(4) American crows and other corvids (blue jays, magpies), passerines, raptors, shorebirds and flamingos are highly susceptible, while domestic poultry and psittacines are generally resistant to fatal infection with WNV.(8) Common histopathological findings in WNV infected wild birds include meningoencephalitis, myocarditis, splenic and bone marrow necrosis; viral antigen is most commonly detected in the kidney, brain and heart.(4) In contrast to the high-titer viremia and widespread necrosis and inflammation found in wild birds, horses tend to have a transient, low-titer viremia with primarily central nervous system involvement.(4) Histological lesions in equids typically include lymphocytic polioencephalomyelitis, predominantly within the lower brain stem and ventral horns of the thoracolumbar spinal cord, with perivascular cuffing and scattered hemorrhage.(2) In addition to wild birds, horses, mules, donkeys and pigs,(5) clinical disease due to WNV has also been reported in reindeer,(9) and Eastern fox squirrels and humans.(5)
Wesselsbron virus, a flavivirus transmitted by Aedes mosquitoes, is important in sub-Saharan Africa, where it causes acute disease resembling Rift Valley fever in sheep.(7) Wesselsbron disease causes high mortality in newborn lambs and kids, with subclinical infection or sporadic abortions in adults.(7)
The mosquito-born flaviviruses described above are typically neurotropic, however yellow fever virus and Dengue virus, which affect humans and monkeys, are viscerotropic, causing hemorrhagic fever rather than encephalitis.(4) Yellow fever virus was originally transported (via its Aedes mosquito vector) to the New World on slave ships, where it decimated the populations of many coastal cities in the 18th and 19th centuries.(7) Disease is biphasic, beginning with fever, headache and nausea, followed by eventual hepatic and renal failure. The widespread icterus often observed with this disease led to the name yellow fever.(7) Old world monkeys tend to have subclinical infections, while New World monkeys develop clinical disease with high mortality.(7) Infection is characterized by widespread midzonal hepatic necrosis, acute renal tubular necrosis and multifocal lymphoid necrosis. Dengue fever is one of the most important viral arthropod-borne hemorrhagic diseases in the world today. African monkeys have been implicated as the original reservoir of the dengue virus, and may still play a role in transmission, however the urban mosquito Aedes aegypti/albopictus is currently the most widely recognized vector in most outbreaks.(7)
References:
2. Cantile C, Del Piero F, Di Guardo G, Arispici M. Pathologic and immunohistochemical findings in naturally occurring West Nile virus infection in horses. Vet Pathol. 2001;38(4):414-421.
3. Endy TP, Nisalak A. Japanese encephalitis virus: ecology and epidemiology. Curr Top Microbiol Immunol. 2002;267:11-48.
4. Kimura T, Sasaki M, Okumura M, Kim E, Sawa H. Flavivirus encephalitis: pathological aspects of mouse and other animal models. Vet Pathol. 2010;47(5):806-818.
5. Kiupel M, Simmons HA, Fitzgerald SD, et al. West Nile virus infection in Eastern fox squirrels (Sciurus niger). Vet Pathol. 2003;40(6):750-752.
6. Mackenzie JS, Barrett, AD, Deubel V. The Japanese encephalitis serological group of flaviviruses: a brief introduction to the group. Curr Top Microbiol Immunol. 2002;267:1-10.
7. MacLachlan NJ, Dubovi EJ, eds. Fenners Veterinary Virology. 4th ed. London, UK; 2011:467-475.
8. Maxie MG, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 5th ed. Vol 1. St. Louis, MO: Saunders Elsevier; 2007:421-422.
9. Palmer MV, Stoffregen WC, Rogers DG, et al. West Nile virus infection in reindeer (Rangifer tarandus). J Vet Diagn Invest. 2004;16(3):219-222.
10. Platt KB, Joo HS. Japanese encephalitis and West Nile virus. In: Straw BE, Zimmerman JJ, DAllaire S, Taylor DJ, eds. Disease of Swine. 9th ed. Ames, IA: Iowa state University Press; 2006:359-365.
11. Shimizu T, Kawakami Y, Fukuhara S, Matsumoto M. Experimental stillbirth in pregnant swine infected with Japanese encephalitis virus. Jpn J Exp Med. 1954;24:363-375.
12. Yamada M, Nakamura K, Yoshii M, Kaku Y. Nonsuppurative encephalitis in piglets after experimental inoculation of Japanese encephalitis flavivirus isolated from pigs. Vet Pathol. 2004;41:62-67.
13. Yamada M, Nakamura K, Yoshii M, Kaku Y, Narita M. Brain lesions induced by experimental intranasal infection of Japanese encephalitis virus in piglets.J Comp Pathol. 2009;141:156-162.