Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 8
October 24, 2018
CASE III: L16-618 (JPC 4083860-00).
Signalment: One juvenile female alligator (Alligator mississippiensis)
History: Out of a cohort of 5,000 alligators, five had decreased appetite but no associated mortalities had been recorded at the time of submission. Alligators of this farm had previously been diagnosed with chlamydia infection. The client also requested testing for West Nile Virus.
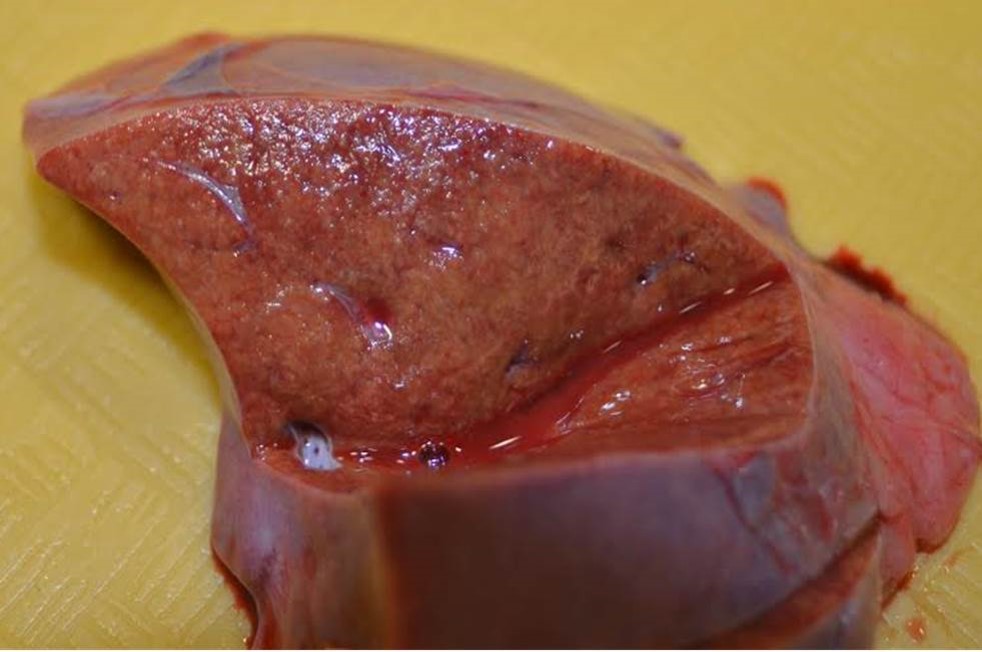
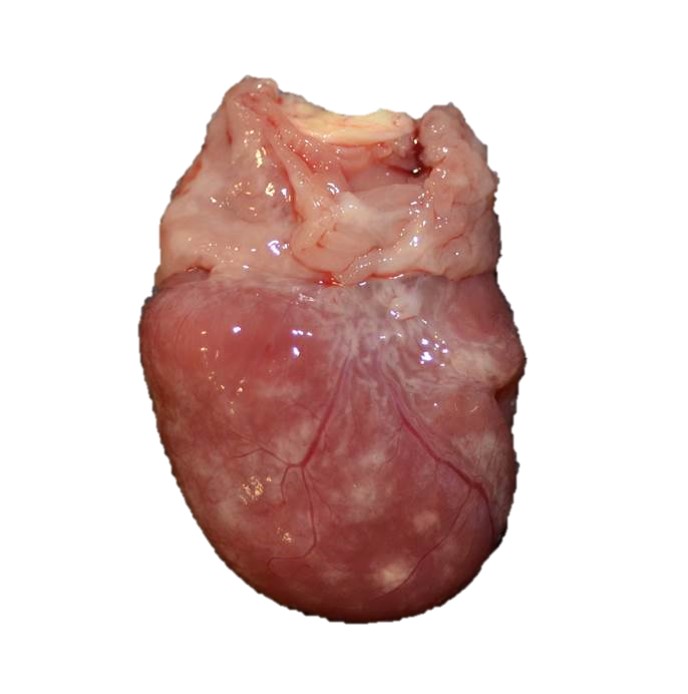
Gross Pathology: The alligator was in good body condition, weighing 2.8 kg. A scant amount of clear free fluid was in the coelomic cavity. The liver was pale tan to green on cut surface . The spleen measured 4 x 1.5 x 1 cm (approximately twice the expected size), was mottled red to dark red, and bulged on cut surface. On the epicardial surface were multiple 1 mm, pale tan foci that did not extend into the myocardium . Minimal red-tinged fluid was in the lower trachea.
Laboratory results:
Molecular (PCR):
Liver, heart, conjunctiva (pooled) and conjunctival swab: Positive for Chlamydiaceae
Liver, kidney, heart, brain, spleen (pooled): Negative for West Nile Virus
Special stains:
Liver: Gimenez-positive microorganisms
Immunohistochemistry
Liver: Microorganisms are immunopositive using a monoclonal anti-chlamydia lipopolysaccharide antibody
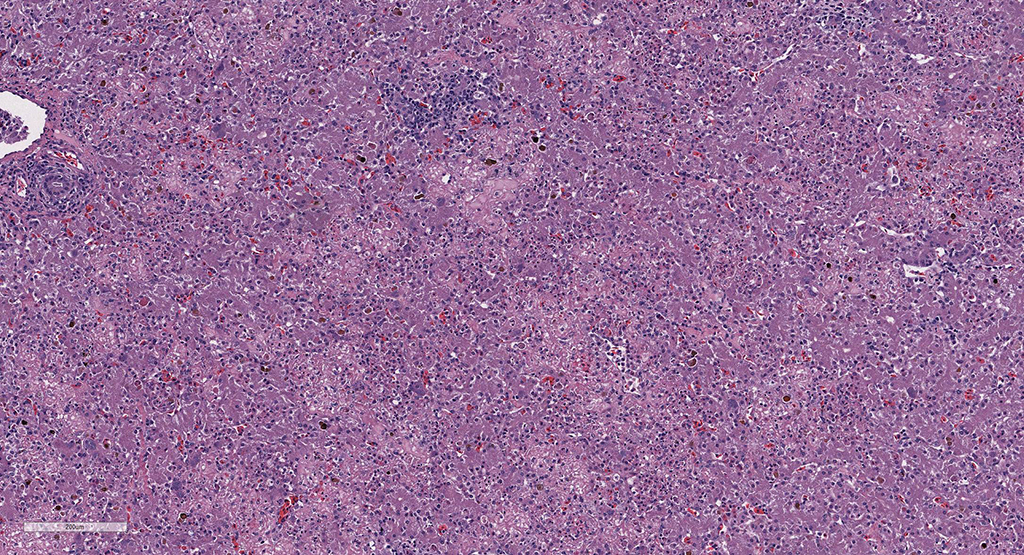
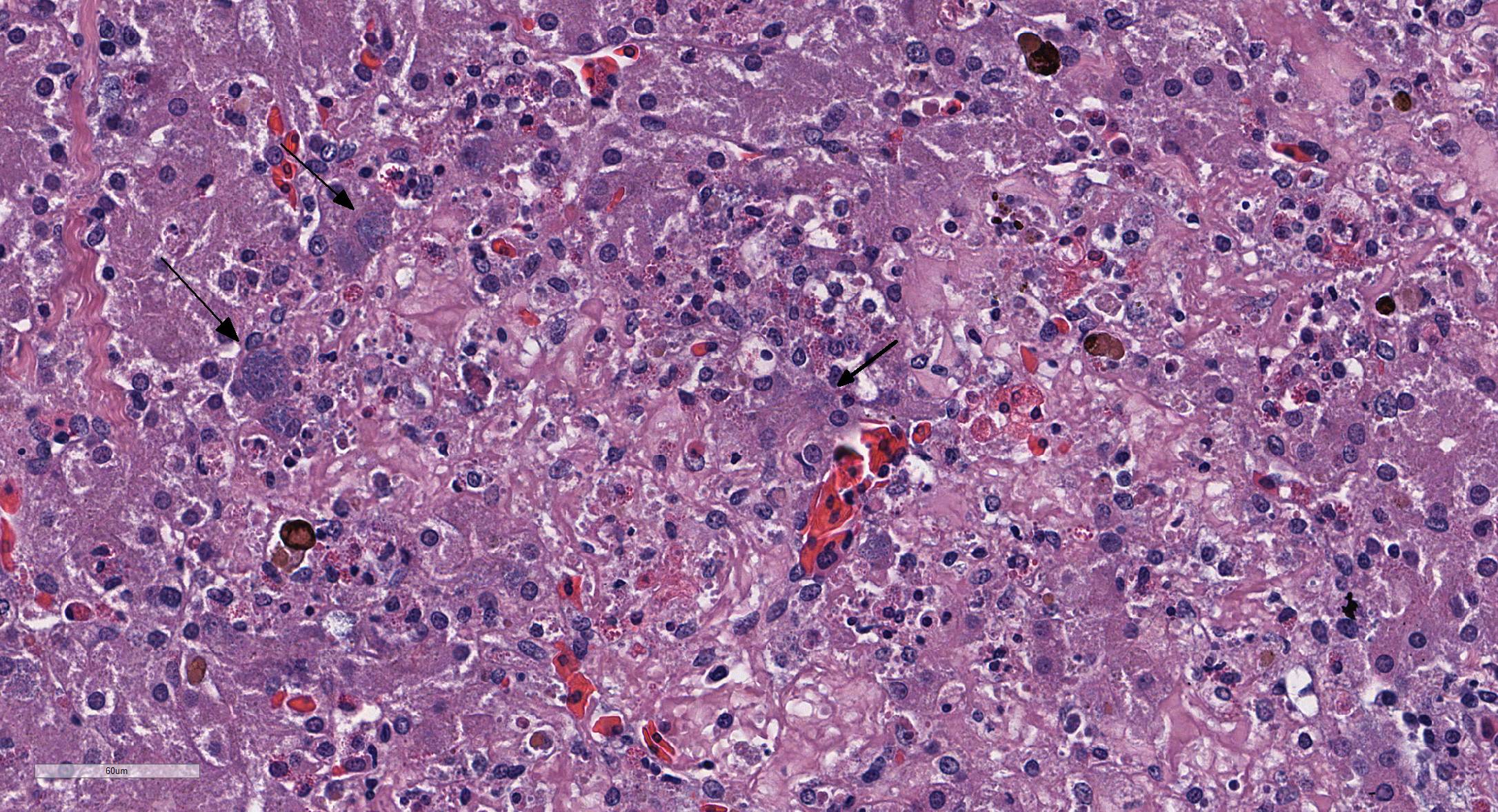
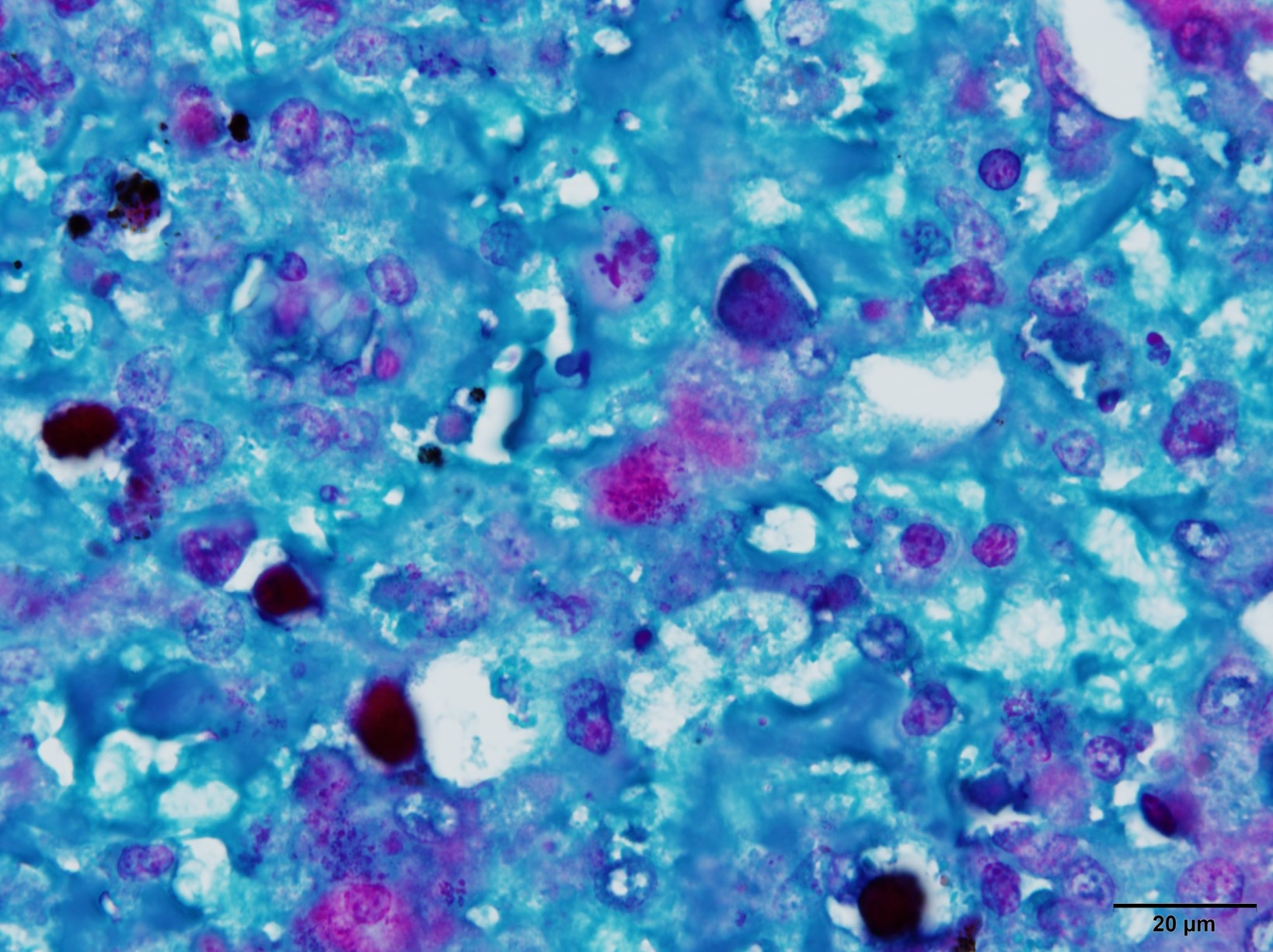
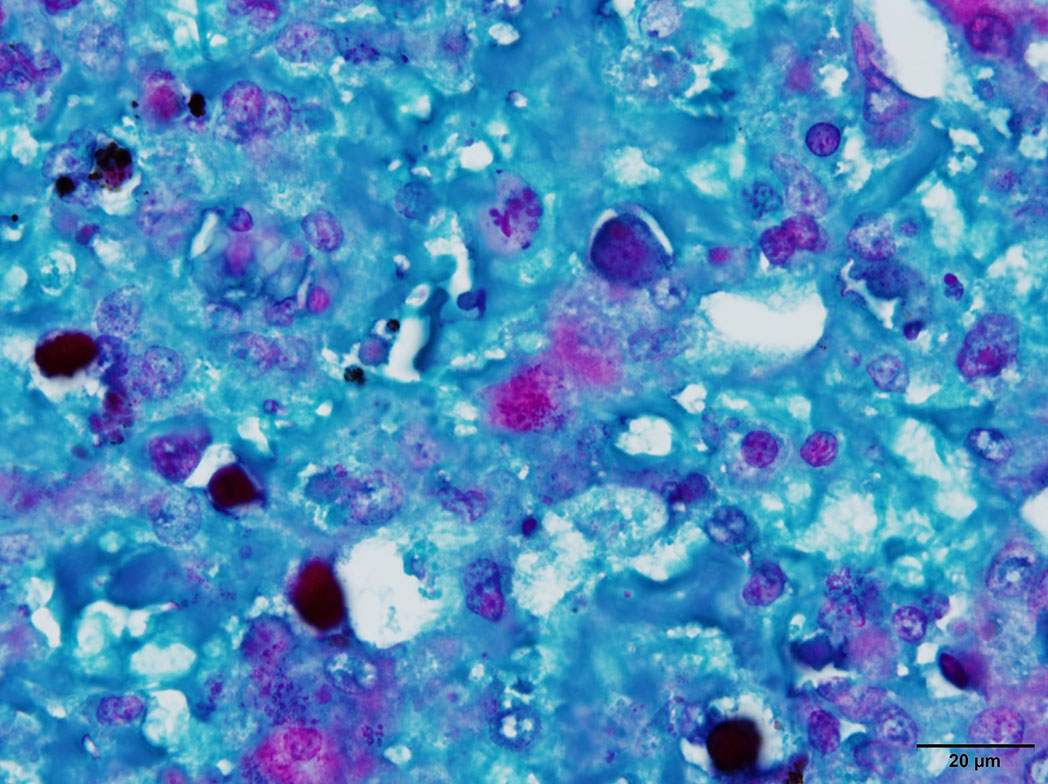
Microscopic Description: Liver: There are are multifocal to coalescing areas of hepatocellular vacuolar degeneration, necrosis, and loss and replacement by aggregates of fibrin, cellular debris, and heterophils. Clusters of basophilic, Gimenez-positive microorganisms are present in multiple remaining hepatocytes. Aggregates of lymphocytes and plasma cells infiltrate portal areas. Biliary epithelial cells, Kupffer cells and, to a lesser extent, hepatocytes contain intracytoplasmic golden yellow-to-dark brown, iron stain-positive and bile stain-negative pigment granules.
Contributor’s Morphologic Diagnoses:
Liver: Hepatitis, necrotizing, multifocal-to-coalescing, marked, acute, with intrahepatocellular coccobacilli
Contributor’s Comment: Chlamydiae represent a phylum of obligate, intracellular bacteria that parasitize an array of hosts including vertebrates, a few arthropod species, and several free-living amebae.2 Ubiquitous in the environment, chlamydiae are further classified into nine different families, all of which share similar developmental cycles and reproductive requirements but differ in their morphology, host specificity and capacity for disease.4 Chlamydiae exist in either one of three stages: an extracellular infectious elementary body (or the dispersal form, analogous to a spore), an intracellular vegetative reticulate body, or, rarely, as described for the Parachlamydiaceae family, a crescent body (also an infective stage).2 The family Chlamydiaceae is comprised of the genera Chlamydia and Chlamydophila. The most significant human pathogens include Chlamydia trachomatis (responsible for urogenital infections and the agent of trachoma) and Chlamydophila pneumoniae (associated with respiratory infections and neurodegenerative syndromes). The majority of the other related species are veterinary pathogens with few anthropozoonotic exceptions, notably Chlamydophila psittaci (psittacosis) and Chlamydophila abortus (spontaneous fetal loss).6 Besides mammals, Chlamydophila also targets birds, amphibians, and reptiles, and chlamydiosis has been described in both free-ranging and captive cold-blooded animals including a variety of snakes, frogs, crocodiles, chameleons, and tortoises.6 Associated lesions in these species are commonly observed in the spleen, heart, lung and liver, characterized by granulomatous inflammation within whichever organ is infected. There are also rare reported incidences of wasting disease; gastrointestinal involvement; and necrotizing myocarditis, enteritis and splenitis.6
The Louisiana Animal Disease Diagnostic Laboratory has recently diagnosed an outbreak of chlamydiosis with high mortalities in a group of farmed American alligators, the first documented report of chlamydiosis in the species.6 A novel reptilian Chlamydophila isolate was identified via PCR with 86% homology to C. psittaci using OmpA gene sequencing. The main pathological findings included necrotizing, heterophilic and granulomatous hepatitis with intracellular bacteria, necrotizing myocarditis, and lymphoplasmacytic, mixed histiocytic and heterophilic conjunctivitis. The alligator of the present submission had similar lesions in both the liver and heart (namely a heterophilic and lymphoplasmacytic necrotizing hepatitis, epicarditis and pericarditis). Additional molecular characterization of this novel Chlamydophila species is warranted to understand its host specificity and infective potential.
In conference, the moderator reviewed some anatomic peculiarities of the reptile liver. The reptile liver is not organized into distinct lobules as in mammals, and hepatic cores are not always seen radially around central veins. Hepatic veins and bile ductules mark the edge of a lobule, and hepatic arteries may or may not be present. She also commented on the difficulty of precisely identifying pigments within the liver of reptiles, which in this case, the attendees variously identified as bile, hemosiderin, or melanin. In reality, many of the aggregates of pigment may stain positively for none, one, or more of these particular pigments on a variety of histochemical stains.
Contributing Institution:
Louisiana Animal Disease Diagnostic Laboratory
http://www1.vetmed.lsu.edu/laddl/index.html
JPC Diagnosis: Liver: Hepatitis, necrotizing, multifocal to coalescing, severe, with rare intrahepatocellular intracytoplasmic bacterial inclusions.
JPC Comment: Prior to any further discussion on this case, it appears (to the delight of pathologists everywhere), all members of the genus Chlamydophila have been replaced in the genus Chlamydia, and the controversial creation of the genus Chlamydophila has been reversed.1
While largely known as an intracellular “energy parasite” of mammals and birds, chlamydiae are also well-established as parasites of reptiles, fish, insects, crustaceans, and bivalves. Chlamydia (or Chlamydia-related bacteria) are the causative agent of “epitheliocystis”, a poorly-named but common parasitic gill disease of fresh and saltwater fish species.1
One of the most interesting parts of the life cycle of chlamydiae is their dependence on host cells for energy. Traditionally, chlamydiae have been considered “energy parasites”, entirely dependent on host cells to supply ATP requirements. Much of this early research was apparently performed on elementary body life stages (a rather metabolically inert form) revealing a lack of flavoproteins and cytochromes associated with traditional mitochondrial function, as well as the presence of two ATP-ADP translocases which allow for energy uptake from the host cell. Recent genomic investigations of other life stages have revealed genetic encoding for many energy pathways, including glycolysis, the Krebs cycle, and the pentose phosphate pathway. Enyzmes in these pathways have been identified in reticulate bodies suggesting a potential for inherent energy creation in more metabolically active life stages than previously thought.5
Another interesting feature of many species of chlamydiae is their symbiotic relationship in the absence of a suitable host with free-living amoeba, to include Acanthamoeba and Hartmanella. While present within the cytoplasm of amoeba, the bacteria are not digested or harmed, and some species may even continue to excrete elementary bodies (the environmentally resistant life stage) into the surrounding extracellular environment. The presence of intracellular chlamydiae have differential effects on the growth rate of various host amebae, and have been documented to increase the cytopathic effects of certain ameba species. In human medicine, combined free-living amebic and chlamydial infections have been documented, likely as a result of infection of susceptible individuals by ameba/chlamydial symbionts.2
Since the submission of this case, a second report of fatal systemic Chlamydia infection in juvenile Siamese crocodiles, with lesions in the heart, liver and spleen similar to those reported in this outbreak, has been published. PCR also suggested a novel Chlamydia sp. The lesions seen in this case, as well as the subsequent report by Thongkamkoon et al., are similar to the lesions seen in estuarine and Nile crocodiles, in which hepatitis is the predominant lesion of systemic chlamydiosis8 and differs from other types of chlamydia-associated disease previously identified in crocodiles, which include infection of the conjunctiva and upper respiratory tract, and death as a result of fibrinous suppurative upper airway and tracheal disease.3
References:
- Borel N, Polkinghorne A, Pospischil A. A review on chlamydial diseases in animals: still a challenge for pathologists: Vet Pathol 2018; 55(3):374-390.
- Corsaro, D. & G. Greub (2006). Pathogenic potential of novel chlamydiae and diagnostic approaches to infections due to these obligate intracellular bacteria. Clinical Microbiology Reviews. 19(2): 283-297.
- Conley KJ, Chilton CM. Crocodilia. In: Pathology of Wildlife and Zoo Animals. Terio K, McAlooseD , St. Leger J, eds. London, Academic Press,. Pp. 849-864.
- Huchzermeyer, F.W., E. Langelet, & J.F. Putterill (2008). An outbreak of chlamydiosis in farmed Indopacific crocodiles (Crocodylus porosus): Clinical communication. Journal of the South African Veterinary Association. 79(2): 99-100.
- Liang P, Rosas-Lemus M, Patel D, Fang X, Tuz K, Juarez O. Dynamic energy dependency of Chlamydia trachomatis on host cell metabolism during different stages of intracellular growth: Possible role of sodium-based energetics in chlamydial ATP generation.
- Sakaguchi, K., Nevarez J, Paulsen D, Bauer R, Crossland N, Langohr I, J. Ferracone J, Ritchi B, Del Piero F . Chlamydiosis in farmed American alligators (Alligator mississippiensis). Poster presented at the 2015 Annual Meeting of the American College of Veterinary Pathologists, Minneapolis, Minnesota, 2015..
- Taylor-Brown, A., S. Rüegg, A. Polkinghorne & N. Borel (2015). Characterisation of Chlamydia pneumoniae and other novel chlamydial infections in captive snakes. Veterinary Microbiology. 178(1–2): 88-93.
- Thongkamkoon P, Tohmee N, Morris EK, Inamniay K, Lombardini ED. Combined fatal systemic Chlamydia and Aeromonas sobria infection in Juvenile Siamese crocodiles (Crocodylus siamensis). Vet Pathol 2018; doi:10.1177/0300985818768382.