Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 25
1 May 2019
CASE III: S-2017-20 (JPC 4100993)
Signalment: 6-year and 5-month old female neutered crossbreed dog (Canis lupus familiaris).
History: A 6-year 5 months-old female neutered Springer Spaniel Cross presented for investigation of pyrexia of unknown origin of 5 months duration. The owner also noted lethargy and mild gastrointestinal signs during this time (inappetence and diarrhoea). Previously the patient had responded well to oral prednisolone and potentiated amoxicillin, however symptoms recurred after the treatment was stopped. On physical examination, marked pyrexia of 40.3C was noted, with tachycardia of 160 beats per minute and normal synchronous pulses. A normal respiratory rate of 32 breaths per minute with normal respiratory effort was noted. Auscultation of the thorax was unremarkable. Mucous membranes were pink and moist, with CRT <2s. Mild discomfort was noted on deep palpation of the cranial abdomen, which was subjectively mildly bloated. Rectal examination was unremarkable and peripheral lymph nodes were within normal limits. Abdominal ultrasound showed free fluid within the abdomen. At exploratory laparotomy the abdomen was diffusely filled with creamy pink fluid and there were multiple masses in the omentum and mesentery of the jejunum, which were sampled for histopathology.
Gross Pathology: In a multifocal to coalescing pattern, the greater omentum was expanded by irregular, tan to brown to dark red and moderately firm mass-like lesions with cream to tan, multifocally red cut surfaces. The adjacent omentum was tan to brown, and soft.
Laboratory results: Haematology: WBC count 18.2x109/L (ref 6-17), otherwise unremarkable. Biochemistry: C-reactive protein 26.3mg/L (ref 0-8.2) and hypoalbuminaemia 17g/L (ref 25-41). ALT was mildly decreased and AST was mildly increased. Abdominal fluid sample: Cytology of aspirated abdominal fluid showed neutrophilic macrophagic inflammation with phagocytosed bacteria and was consistent with a septic exudate. Culture of omental masses: Actinomyces spp. was cultured.
Microscopic Description:
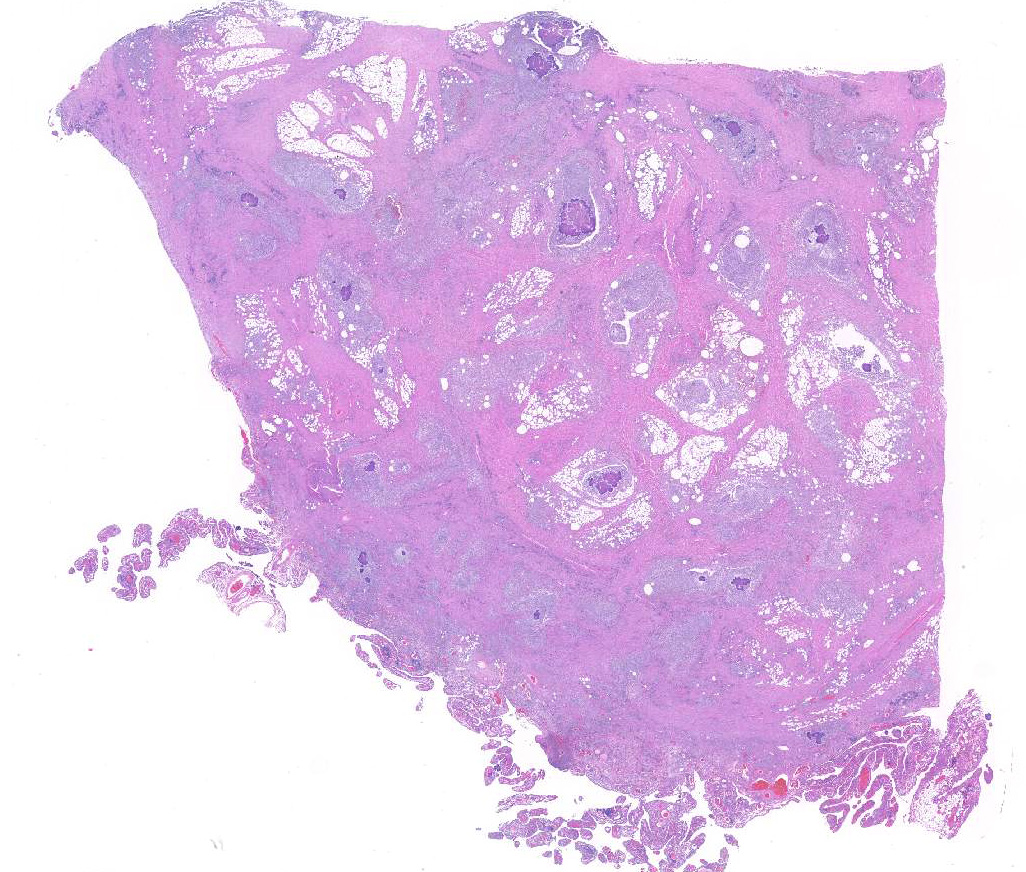
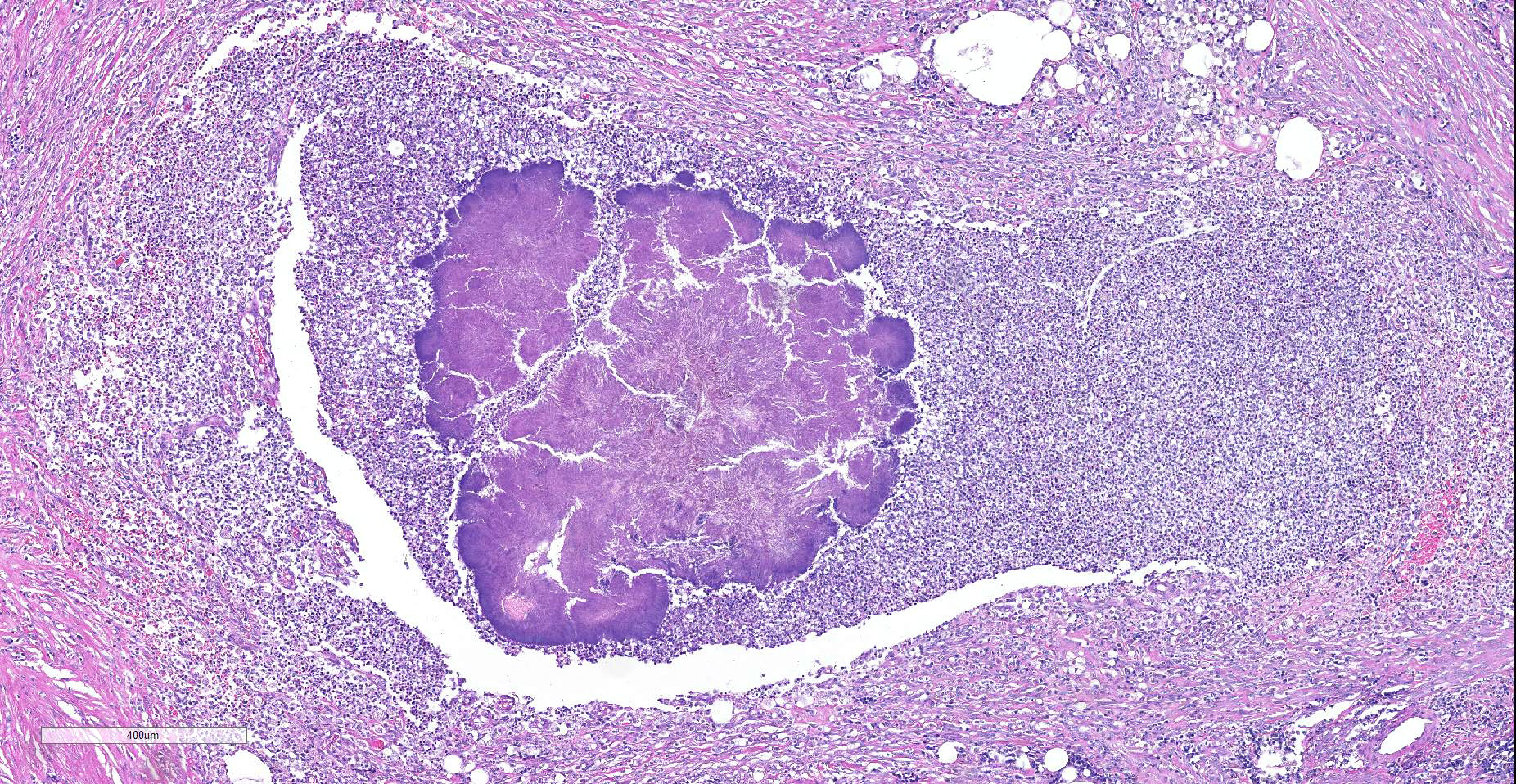
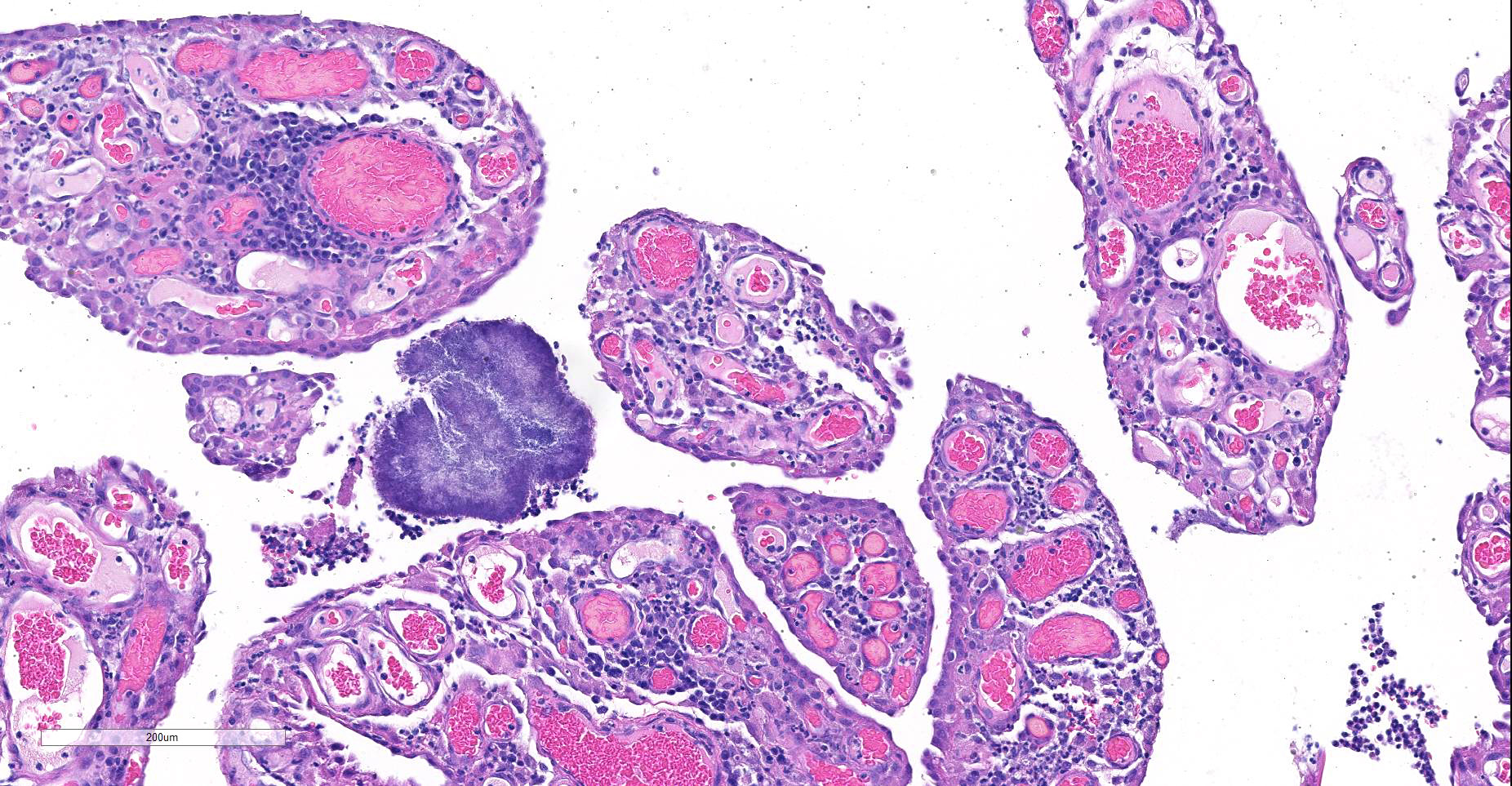
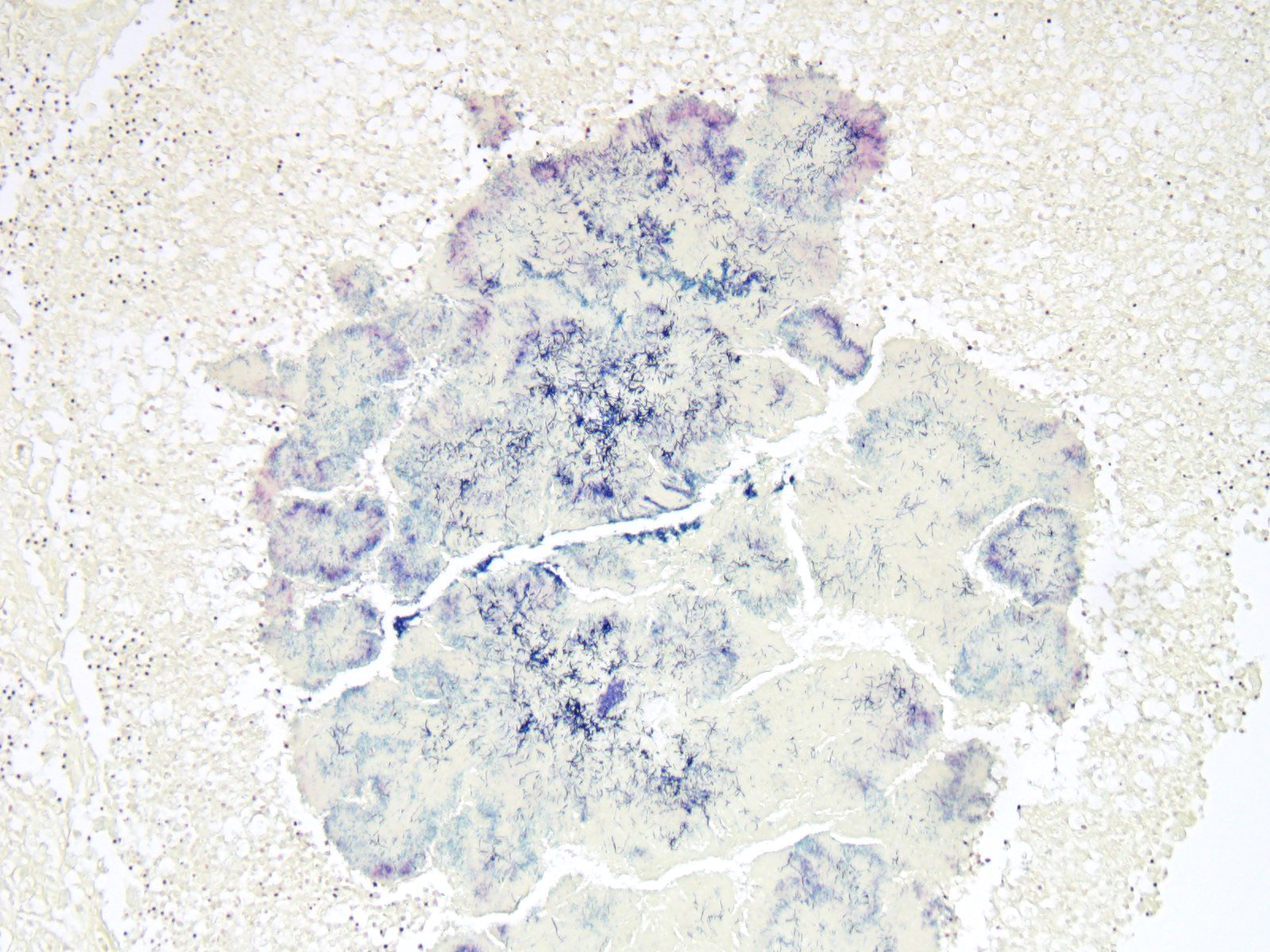
Expanding and effacing the collagenous connective tissue and adipose tissue of the omentum, in a multifocal to coalescing distribution, are large numbers of macrophages, neutrophils and lesser numbers of lymphocytes, which are often forming moderately sized to large groups. Small to moderate numbers of plasma cells are present multifocally, often at the periphery of these groups and multifocally within the connective tissue and adipose. Large numbers of macrophages and neutrophils often surround large colonies of filamentous Gram-positive bacteria, measuring approximately 1x3-7µm. Bacteria are admixed with large (approximately 500µm diameter) accumulations of finely granular basophilic to eosinophilic material which radiates outwards from a central focus (Splendore-Hoeppli material; “sulphur granules”). These colonies often show a rosette-like arrangement and are visible subgrossly. Small amounts of eosinophilic material, cellular debris, haemorrhage and small numbers of haemosiderin-laden macrophages (haemosiderophages) multifocally surround the colonies. Further to the periphery of these foci are haphazardly arranged, hypertrophied fibroblasts embedded in fibrous connective tissue which often progresses to thick bands of mature fibrous connective tissue, containing scattered to moderate numbers of neutrophils, lymphocytes, plasma cells, and fewer macrophages.
The omentum is lined by flattened to multifocally plump mesothelial cells and exhibits multifocal ulceration. Underlying ulcerated areas there are frequent plump reactive fibroblasts, aligned in parallel to each other and perpendicular to adjacent, newly formed blood vessels (angiogenesis) and to the ulcerated surface (granulation tissue formation). At the edges of the section the omentum exhibits a multifocal folded appearance and contains numerous, frequently congested, blood vessels and is expanded by variable numbers of macrophages, lymphocytes, neutrophils, plasma cells, and fibroblasts.
Contributor’s Morphologic Diagnoses:
Greater omentum: Peritonitis, pyogranulomatous, lymphocytic, and plasmacytic, chronic, locally extensive, severe; with:
- Numerous accumulations of Splendore-Hoeppli material with intralesional microcolonies of Gram-positive filamentous rod bacteria, morphology consistent with Actinomyces spp.;
- Granulation tissue formation, chronic, multifocal, marked; and
- Fibrosis, chronic, multifocal, marked.
Contributor’s Comment: The histopathological findings in this case were consistent with abdominal actinomycosis, a diagnosis that was supported by the microbiology results. The patient had an abdominal drain placed at the time of exploratory laparotomy and was subsequently hospitalized for 6 days. Antibiotic treatment with amoxicillin-clavulanic acid commenced following receipt of the histopathology and culture results, and treatment resulted in good clinical improvement and a positive outcome.
Actinomycosis is caused by filamentous, gram-positive bacteria from the family Actinomycetaceae, genus Actinomyces, and to a lesser extent to the related genus Arcanobacterium. These bacteria are normally present in the mucous membranes, especially in the oropharynx, and in the genital and gastrointestinal tracts, having however the potential to cause opportunistic infection when inoculated into tissues in association with other bacteria when there is mechanical disruption of normal mucosal barriers (such as deeply penetrating wounds or migration of foreign material).7,14
Canine actinomycosis is most common in young adult to middle-aged large breed dogs (median age of 5 years old) that have outdoor access, especially retriever and hunting breeds. The development of actinomycosis in outdoor dogs is frequently related to exposure to plant penetrating material, such as grass awns.7,14 After ingestion or inhalation, plant awns become contaminated with Actinomyces spp. and other bacteria from the oropharynx, and then migrate to various sites.6,7,14
Actinomyces spp. can also be inoculated into tissues by bite injury. Other co-infecting aerobic and anaerobic bacteria from the oral cavity or intestinal tract impair normal host defenses and reduce oxygen tension, which allows Actinomyces spp. to endure.7,14 Abdominal actinomycosis, as seen in this case, may develop when ingested foreign bodies penetrate the gastrointestinal tract, which leads to the formation of intra-abdominal mass lesions and ascites or it can also occur due to direct extension from subcutaneous tissues or hematogenous spread of the organism to abdominal organs such as the liver.9,14
Actinomyces spp. that have fimbriae can bind to specific cell surface receptors on other bacteria, especially streptococci, and this bacterial co-aggregation prevents the capacity of neutrophils to phagocytize the organisms.13,14 Dense colonies of Actinomyces spp. form, and these ´Sulfur’ granules can be macroscopically visible in exudates in actinomycosis as small yellow free granules.15 The colonies are progressively encircled by concentric accumulations of neutrophils, macrophages, and plasma cells, with the ensuing development of pyogranulomatous inflammation14 and are often bordered by star-like or club-shaped-like amorphous eosinophilic material (Splendore-Hoeppli material).
The connective tissue is destroyed by proteolytic enzymes from the associated bacteria, macrophages and degranulated neutrophils, which allows the inflammatory process to extend through normal tissue planes. Less frequently Actinomyces spp. spreads hematogenously to distant sites. In some cases, the inflammatory reaction is accompanied by mass formation and extensive fibrosis, as in this case. The most common clinical forms of actinomycosis in dogs and cats involve the cervicofacial region, thorax, abdomen, and subcutaneous tissue, but central nervous system infections including meningitis and meningoencephalitis,5,14 and ocular infections such as keratitis and endophthalmitis,3 may also occur.
In cattle, actinomycosis, caused by Actinomyces bovis, often affects the bones of the jaw, with development of granulomatous and fibrosing osteomyelitis (‘lumpy jaw’). Less commonly it can also affect the musculature of the tongue, leading to the development of chronic fibrosing nodular myositis.16 Actinomyces spp. may also cause hepatic abscesses in different species.4 In swine, Actinomyces suismastidis has been associated with the development of pyogranulomatous mastitis10 and Actinomyces hyovaginalis has been associated with necrotic pulmonary lesions in the same species.2 In horses, Actinomyces spp. can cause abscesses, which are most commonly located in the submandibular and retropharyngeal regions8 and Actinomyces denticolens has been associated with the development of submandibular lymphadenitis.1
Contributing Institution:
Department of Veterinary Medicine, University of Cambridge, Madingley Road, Cambridge CB3 0ES, UK. http://www.vet.cam.ac.uk/
JPC Diagnosis: Omentum: Peritonitis and steatitis, chronic-active and pyogranulomatous, multifocal to coalescing, severe, with large colonies of filamentous bacilli and marked mesothelial hyperplasia
JPC Comment: The contributor has provided an excellent review of actinomycotic infection in the dog and a wider range of species. Actinomyces is a genus of gram-positive bacteria, with new species being identified on a regular basis in species as diverse as mammals and mollusks.
Members of the genus Actinomyces are well documented in health and disease in humans as well. A number of species of Actinomyces, including A. odontolyticus, A. oris, and A. naeslundii, are common commensals in the oral cavity, and are components of the biofilm on teeth at all ages. A number of commensal actinomycetes colonize the gastrointestinal tract, where they are proposed to help in the breakdown of complex sugars as well as producing antibiotics to maintain balance of the bacterial flora.12
The pathology of actinomycosis in humans was first identified in 1892 by Kruse, when the bacterium was known as Streptomyces israelii (now A. israelii).11 Twenty-five species of pathogenic actinomycetes have now been identified in humans. These species of Actinomyces are endogenous inhabitants of mucosal membranes which are introduced into deeper tissues by trauma, surgery, or the introduction of foreign bodies. In humans, actinomycosis is classified as orocervicofacial, thoracic, and abdominal. More than half of all cases of human actinomycosis are orocervicofacial, an unsurprising fact considering the ubiquity of multiple species in the oral cavity, and many infections are polymicrobial in nature. Unlike dogs, the basis for thoracic infection is usually aspiration of oropharyngeal secretions, and results in pulmonary abscessation. Extrapulmonary extension into the thorax (common in small animals) is relatively uncommon in humans, with sepsis being a more common result. Abdominal infections are often the result of abdominal surgery or invasive infection such as appendicitis; pelvic infections have been associated with prolonged use of intrauterine devices for contraception. Less common manifestations are cutaneous and musculoskeletal infections (generally caused by traumatic implantation, and cerebral and disseminated actinomycosis (usually resulting from sepsis). An excellent review of human infection, to include specific forms of infection and associated actinomycotic species was published by Kononen et al. in 2015.11
The moderator discussed the nature of Splendore-Hoeppli material and various theories concerning its makeup, and possible mechanisms of infection the dog and cat, as well as actinomycotic infections in other species.
References:
- Albini S, Korczak BM, Abril C, et al. Mandibular lymphadenopathy caused by Actinomyces denticolens mimicking strangles in three horses. Vet Rec. 2008;162(5):158-9.
- Aalbæk B, Christensen H, Bisgaard M, et al. Actinomyces hyovaginalis Associated with disseminated necrotic lung lesions in slaughter pactinomyces veterinary igs. J Comp Pathol. 2003;129 (1):70–77.
- Barnes LD, Grahn BH. Actinomyces endophthalmitis and pneumonia in a dog. Can Vet J. 2007;48(11):1155-8.
- Brown DL, Van Wettere AJ, Cullen JM – Hepatobiliary System and Exocrine Pancreas In: Zachary FJ, ed. Pathologic Basis of Veterinary Disease: Elsevier Saunders, 2017:442.
- Couto SS, Dickinson PJ, Jang S, et al. Pyogranulomatous meningoencephalitis due to Actinomyces sp. in a dog. Vet Pathol. 2000;37(6):650-2.
- Edwards DF, Nyland TG, Weigel JP. Thoracic, abdominal, and vertebral actinomycosis. Diagnosis and long-term therapy in three dogs. J Vet Intern Med. 1988;2(4):184-91.
- Edwards DF. Actinomycosis and nocardiosis. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 1st ed. Philadelphia: WB Saunders, 1990:585–590.
- Fielding CL, Magdesian KG, Morgan RA, Ruby RE, Sprayberry KA. Actinomyces species as a cause of abscesses in nine horses. Vet Rec. 2008;162(1):18-20.
- Gavhane, D.S., Moregaonkar, S.D., Mhase, A.K., Sawale, G.K. and Kadam, D.P.A Case of Chronic Abdominal Actinomycosis with Severe Hepatic Involvement in a Dog. Israel J Vet Med. 2016; 71(1):58.
- Hoyles L, Falsen E, Holmström G, et al. Actinomyces suimastitidis sp. nov., isolated from pig mastitis. Int J Syst Evol Microbiol. 2001;51(Pt 4):1323-6.
- Kononen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev doi: 10.1128/CMR.00100-14.
- Li J, Li Y, Zhou Y, Wang C, Wu B, Wan J. Actinomyces and alimentary tract diseases a review of it. Biomed Res Int 2018; https://doi.org/10.1155/2018/3820215
- Ochiai K, Kurita-Ochiai T, Kamino Y, et al. Effect of co-aggregation on the pathogenicity of oral bacteria. J Med Microbiol. 1993;39(3):183-90.
- Sykes J. Bacterial diseases - Actinomycosis In: Sykes J, ed. Canine and Feline Infectious Diseases. St Louis, MO: Elsevier Saunders, 2014:399-408.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Pathology of Domestic Animals, Volume 2. 6th ed. St Louis, MO: Elsevier, 2015:253.
- Valentine B – Skeletal Muscle In: Zachary FJ, ed. Pathologic Basis of Veterinary Disease. 6th ed. St Louis, MO: Elsevier Saunders, 2017:926, 942.