Signalment:
Gross Description:
Histopathologic Description:
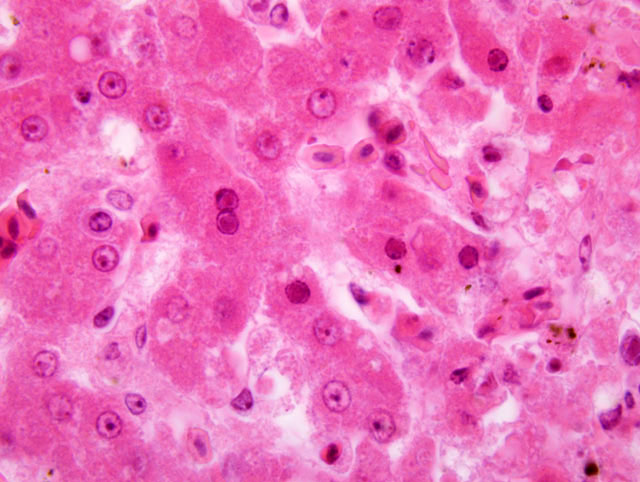
Spleen: The parenchyma is replaced by multifocal to coalescing areas of acute necrosis, partly associated with acute haemorrhages resulting in complete loss of the typical architecture of the organ. Several reticuloendothelial cells of the white pulp closely adjacent to these regions contain eosinophilic intranuclear inclusion bodies (Cowdry A-type). A beginning demarcation of necrotic regions by neutrophilic granulocytes is seen in some areas of the spleen. The unaffected white pulp is highly depleted. Furthermore, numerous histiocytes are filled with a brown, chunky pigment (haemosiderocytes). Frequently, areas with an accumulation of dark brown pigment that appears green at the borders can be seen (formalin-pigment). The capsule of the organ is normal.
Morphologic Diagnosis:
1. Liver: moderate, acute, multifocal sometimes coalescing necrosis, with multifocal eosinophilic intranuclear inclusion bodies, Herpesviridae.
2. Spleen: severe, acute, multifocal to coalescing necrosis, with multifocal eosinophilic intranuclear inclusion bodies, Herpesviridae.
Lab Results:
Lungs: Aspergillus spp.; coagulase-negative Streptococcus spp.; and Escherichia coli.
Liver and spleen: no bacterial growth observed
Extracts from liver and spleen were cultured on chicken-embryo-hepatocytes and chicken-embryo-fibroblasts in cell culture and the supernatant was analyzed on an electron microscope. These examinations revealed Herpesviridae in both organ samples.
Condition:
Contributor Comment:
The family of avian herpesviruses comprises three subfamilies, including alpha-, beta- and gamma-Herpesviruses. Each name for the genera derives from the predominant clinical and pathological findings.(10) Hepatosplenitis infectiosa strigum (HSIS) is caused by the strigid herpesvirus-1 (SHV-1) and is dedicated to the betaherpesviruses (synonym hepatosplenitis viruses). This disease occurred in 1915 in Austria for the first time (retrospective examination of fixed tissue samples of affected birds), (3) but was first described in the United States in 1936.(8) It is seen frequently in Germany since 1969.(4,12) The SHV-1 is a strict host-specific pathogen that reveals close similarities to the falconid herpesvirus-1 (FHV-1) and the columbid herpesvirus-1 (CHV-1). Due to the large genomic homology, a PCR styled to detect the CHV-1 can also be used to detect SHV-1 and FHV-1.(1) Reported susceptible species of owls for HSIS are: the Eagle Owl (Bubo bubo), the Great Horned Owl (Bubo virginianus), the Striped Owl (Asio clamator), the Long-eared Owl (Asio otus), the Snowy Owl (Nyctea scandiaca), the Little Owl (Athene noctua), and the Boreal Owl (Aegolius funereus), whereas the Eurasian Tawny Owl (Strix aluco) and the Barn Owl (Tyto alba) seem to be naturally resistant.(4) However, other authors have shown that American kestrels, budgerigars and ring-necked doves are susceptible for experimental infections, as well.(12)
Additionally, evidence for latent infections was found, given the fact that antibody-positive but healthy animals could be observed.(9) As reported by Burtscher and Sibalin,(4) only owls with a yellow or orange coloured iris (of the aforementioned species) have proven to be susceptible, whereas species with dark irises (e.g. Tawny Owls and barn owls) were resistant, even to massive experimental infections. However, the authors considered this finding not to be significant, but remarked further investigations were needed to clarify.
The virus has a tropism for mesenchymal cells and to a lesser extent epithelial cells.(2,4) An oropharyngeal route of infection is assumed. Virus-shedding takes place through the pharynx and urine. After an incubation period of 7 to 10 days, affected animals show apathy, anorexia and ruffled feathers. At later stages, they support themselves with their wings standing on the soil and fall into the prone position in which they are perishing in the following period. (2,8,11)
As in the present case, findings during necropsy are: good nutritional condition, swelling of the liver (hepatomegaly) and pale white foci of caseous necrosis in the liver, spleen and bone marrow (not recorded in this case). Microscopic examinations reflect the necrotic regions to be mostly without any inflammatory reaction, presumably due to the fulminant clinical progress of the disease. Viable and degenerating hepatocytes, particularly at the edges of the necrotic foci, show intranuclear eosinophilic inclusion bodies (Cowdry A-type).(2,8,11)
It was not possible to establish the source of the infection in this case. Even intense anamnestic survey could not identify potential reservoirs (no acquisition of animals, no contact to wildlife animals and no changes in the acquisition of prey animals). Regardless, no further infections were recorded in this aviary. This might be due to the strict hygienic prevention (including the prey animals) that was performed acting upon the advice of the private veterinarian and the Institute of Pathology of the University of Leipzig.
JPC Diagnosis:
1. Liver: Hepatitis, random, necrotizing, acute, multifocal, moderate, with hepatocellular intranuclear eosinophilic inclusion bodies.
2. Spleen: Splenitis, necrotizing, acute, multifocal to coalescing, marked with intranuclear eosinophilic inclusion bodies.
Conference Comment:
Some discussion focused on the epidemiology of HSIS in wild birds of prey. The moderator mentioned that this disease is only seen in wild, and not captive, falcons and owls. Recent research demonstrated that the herpesviruses isolated from owls, falcons, hawks and pigeons were all identical. The authors concluded that owls, falcons and hawks are infected with the same virus, Columbid herpesvirus-1, and therefore captive birds of prey should not be fed pigeons.7
The moderator commented that a few avian herpesviruses are associated with neoplasia. One example, Psittacid herpesvirus-1, the etiology of Pachecos disease, is known to cause psittacine papillomatosis; gross findings are papillomas in the choana and cloaca.(14) The herpesviral-induced proliferative epithelial lesions can undergo malignant transformation to squamous cell carcinoma or adenocarcinoma. More recently, DNA from this virus was detected in a pancreatic duct carcinoma in a macaw.(13)
References:
2. Burki F, Burtscher H, Sibalin M. Herpesvirus strigis: a new avian herpesvirus. I. Biological properties. Arch Gesamte Virusforsch. 1973;43:14-24.
3. Burtscher H. Die virusbedingte Hepatosplenitis infectiosa strigum. 1. Mitteilung: Morphologische Untersuchungen. Pathol Vet. 1965;2:227-255.
4. Burtscher H, Sibalin M. Herpesvirus strigis: host spectrum and distribution in infected owls. J Wildl Dis. 1975;11:164-169.
5. Cheville NF. Response to cellular injury. In: Ultrastructural Pathology: The Comparative Basis of Cellular Disease. 2nd ed. Ames, IA: Wiley-Blackwell; 2009:13.
6. Cheville NF. The neoplastic cell. In: Ultrastructural Pathology: The Comparative Basis of Cellular Disease. 2nd ed. Ames, IA: Wiley-Blackwell; 2009:7665
7. Gailbreath KL, Oaks JL. Herpesviral inclusion body disease in owls and falcons is caused by the pigeon herpesvirus (Columbid herpesvirus 1). J Wildl Dis. 2008; 44(2):427-433.
8. Green RG, Shillinger JE. A virus disease of owls. Am J Pathol. 1936;12:405-410.
9. Kaleta EF, Dr+â-+ner K. Hepatosplenitis infectiosa strigum und andere Krankheiten der Greifv+â-¦gel und Eulen. Zentralblatt Vet Med. 1976;25(Suppl):173-180.
10.Kaleta EF. Herpesviruses of birds: a review. Avian Pathol. 1990;19:193-211.
11.Lee LF, Armstrong RL, Nazerian K. Comparative studies of six avian herpesviruses. Avian Dis. 1972;16:799-808.
12.Mare CJ. Herpesviruses of birds of prey. J Zoo Animal Med. 1975;6:6-11.
13.Mundhenk L, M+â-+ller K, Lierz M, et al. Psittacid herpesvirus DNA in a pancreatic duct carcinoma in a macaw. Vet Rec. 2009;164:306-308.
14.Styles DK, Tomaszewski EK, Jaeger LA, Phalen DN. Psittacid herpesviruses associated with mucosal papillomas in neotropical parrots. Virology. 2004;325(1):24-35.