WSC 2023-2024, Conference 19, Case 3
Signalment:
5-year-old male porcupine (Erethizon dorsatum)
History:
This porcupine was born in captivity and transferred to another zoo one year later. The animal showed the following clinical signs: apathy, shortness of breath, and ataxia. The
clinical examination revealed a body temperature of 33.2°C. The animal died shortly after the onset of symptoms.
Gross Pathology:
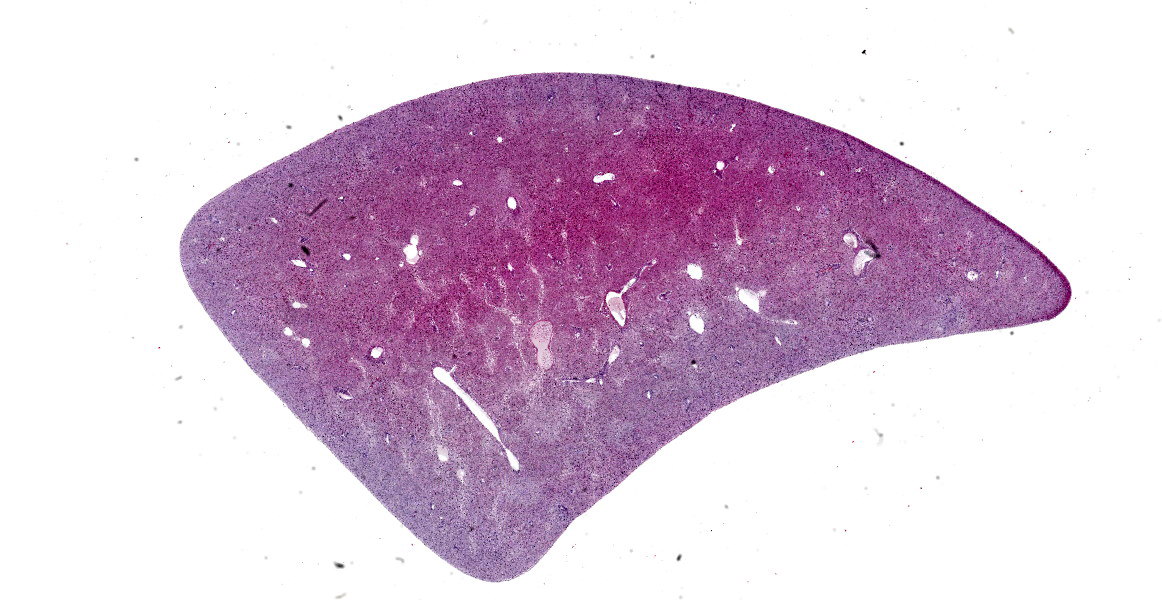
Liver: On gross examination, the liver was predominantly wine-red, with some black-red areas. The liver margins were partly blunt-edged. The cut surface was demarcated, wine-red and firm. Furthermore, there were several to abundant beige-coloured, soft, sharply demarcated foci with a diameter of 1 to 5 mm.
Microscopic Description:
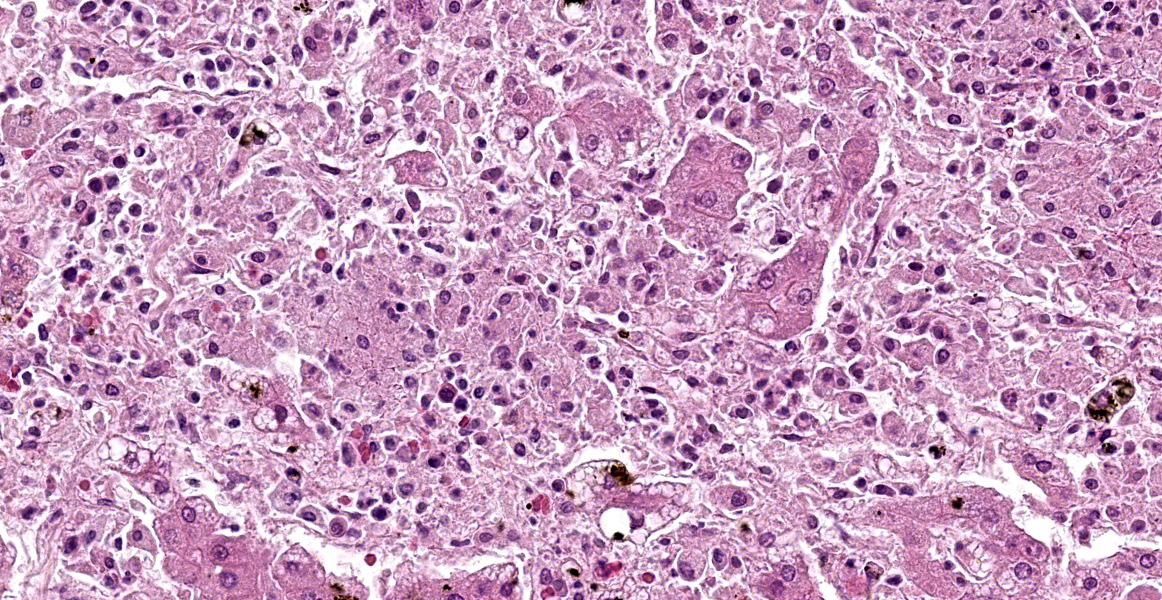
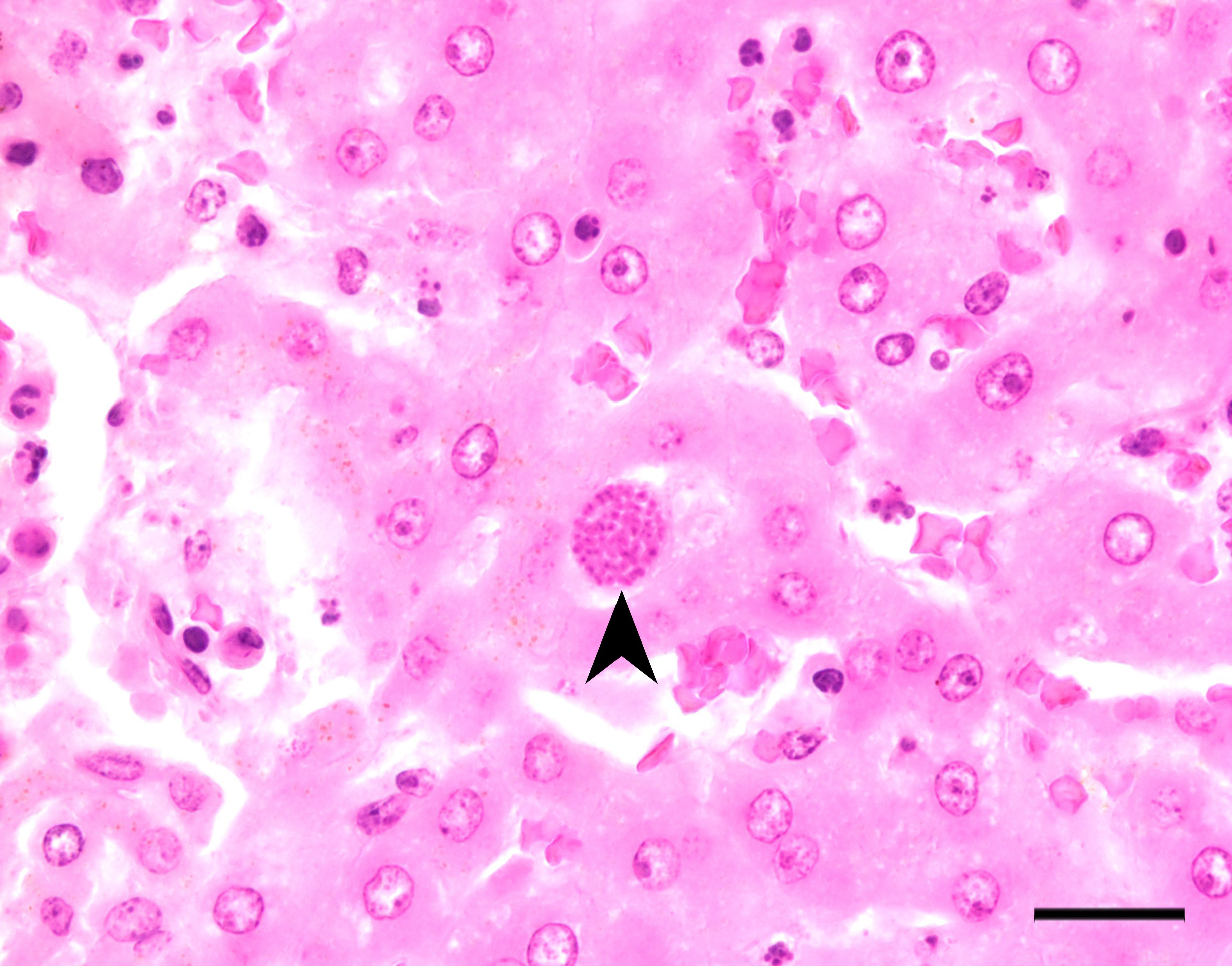
Liver: There are multifocal, randomly distributed hypercellular foci affecting 30% of the liver parenchyma. The foci consist of many macrophages, lymphocytes, and few neutrophils. Scattered hepatocytes display cytoplasmic swelling, pallor, membranous rupture, nuclear pyknosis and karyorrhexis (lytic necrosis). Within the inflammatory foci there are rare intrahepatocytic, intracytoplasmic, 15-20 µm in diameter, oval apicomplexan cysts with a thin capsule and more than 20, 1-2 µm, basophilic, banana-shaped bradyzoites, and rare extracellular, 1-2 µm, basophilic, oval tachyzoites. Portal areas are expanded by 2-5 layers of lymphocytes, macrophages, and plasma cells. There is minor and variably distributed bile duct hyperplasia and portal fibrosis.
Inflammatory changes with variable severity were found in the heart, lungs, intestines, spleen, urinary bladder, and brain (not on submitted slide).
Contributor’s Morphologic Diagnosis:
Liver: Hepatitis, lymphohistioplasmacytic and necrotizing, multifocal, random, subacute, moderate with intralesional apicomplexan cysts.
Contributor’s Comment:
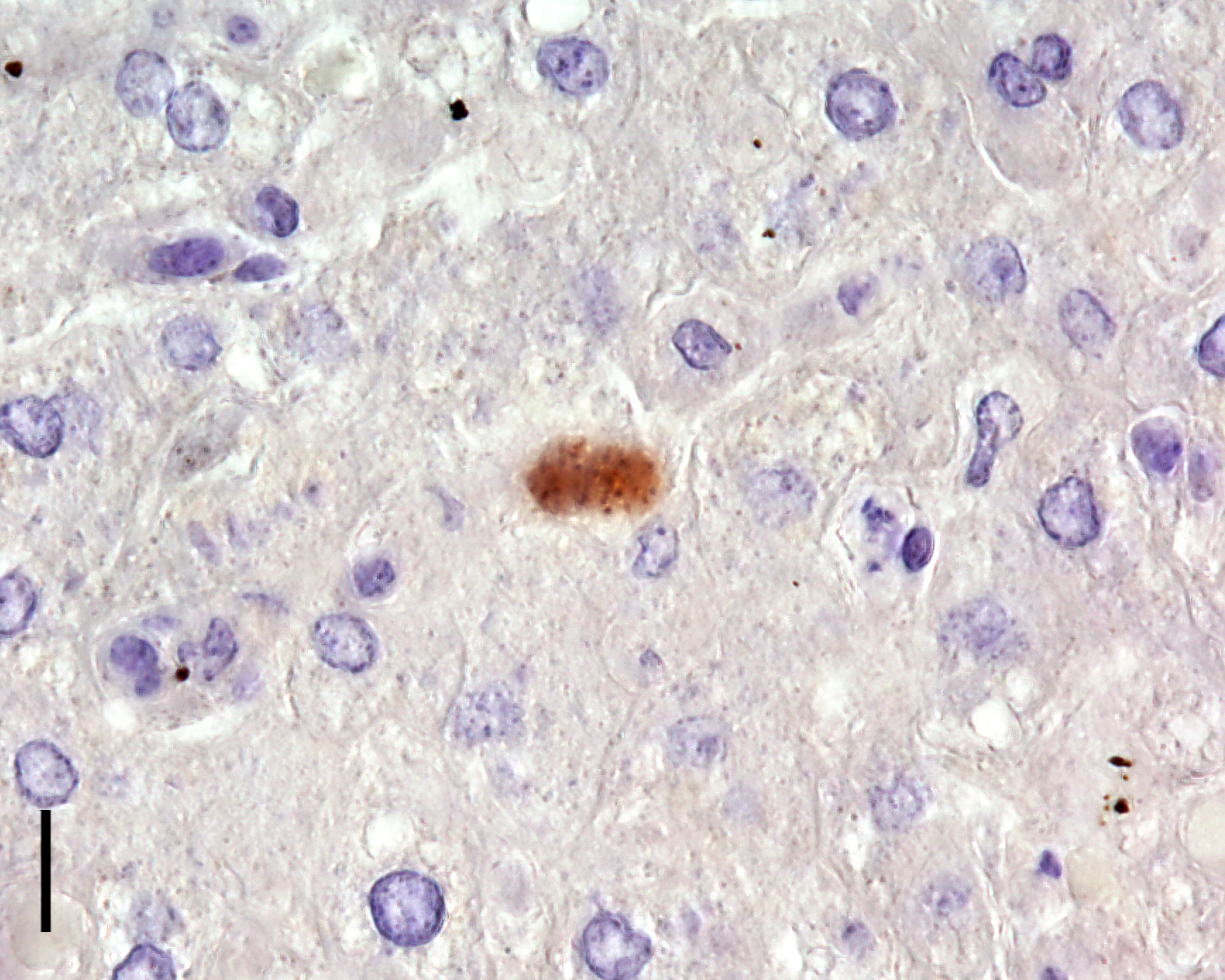
Due to the typical histomorphological findings, an infection with Toxoplasma gondii (T. gondii) was suspected as the cause of the lesions in the present case. Immunohistochemistry was performed and T. gondii was verified as the causative agent.
Toxoplasmosis is one of the most common and widespread diseases in humans and warm-blooded animals worldwide.10 In 1908, the parasitic pathogen was first isolated from a gundi (Ctenodactylus gundi) in Tunis and a rabbit from South America.1 The genus toxoplasma contains T. gondii as the only species, which can be divided into 189 different genotypes.1,3 The epidemiological and pathogenetic significance depends on the respective genotypes. In the northern hemisphere, especially in Europe, the most common genotypes are 1 and 3. Genotypes 2 and 5 are mainly isolated in North America and genotypes 2 and 3 in Africa. In China, genotypes 9 and 10 represent the majority of isolates.1 T. gondii has a very broad host spectrum; thus, numerous warm-blooded animals as well as humans are susceptible as intermediate host. Cats and wild felids serve as final hosts.
Epidemiology: Approximately one third of the world's human population is infected with T. gondii, although prevalence varies from region to region. In the USA and the United Kingdom, about 16 to 40% of people are infected.7,9 In contrast, the infection rate in Central and South Africa and continental Europe is 50 to 80%.9 Evidence of infection was found in 31 of the world's 39 felid species.10 Prevalence in wildlife animals depends on various physical, biological, and ecological factors as well as climatic conditions and the susceptibility of host species.10 The prevalence in marine mammals (e.g., seals, sea otters and dolphins) is particularly interesting, as it ranges from 47 to 100%. There have been some reports of toxoplasmosis in New World porcupines emphasizing this species as susceptible to the disease. Numerous comparable cases of toxoplasmosis have been described in various animals in zoologic gardens, including New World primates, Australian marsupials, and Pallas’ cats.2 Toxoplasmosis is an important cause of sporadic and epizootic mortality in zoo populations. In most cases, the affected animals are over 12 months old. Old world monkeys, rats, cattle, and horses seem highly resistant to infection. 1,2
Pathogenesis/development cycle: T. gondii exhibits facultative heteroxenic development.3,12 Depending on the position in the developmental cycle, different morphological stages of T. gondii occur.8 Tachyzoites represent slightly curved, crescent-shaped cells with an apical complex at the anterior pole and a nucleus in the posterior half of the cell. The cell contains one chromosomal and one mitochondrial genome as well as a genome of circular DNA. Outside the host cell, the tachyzoites are only viable for a short period of time and are destroyed during gastric passage. During endodyogeny, bradyzoites are formed within the cyst lumina. The cysts, which are up to 150 µm in size, develop intracellularly in various tissues and, due to their resistant wall, have a relatively long life span in the host. A cyst can carry up to several thousand bradyzoites. The oocyst contains two sporocysts with four sporozoites each. Overall, development in the final and intermediate host proceeds along different paths, whereby only nucleated cells are infected. Both intermediate and definitive hosts can become infected under natural conditions mainly via three infectious routes: oral ingestion of oocysts from the environment (horizontal); oral ingestion of cysts within the tissues of intermediate hosts (horizontal); and diaplacental or galactogenic transmission of tachyzoites (vertical).
A particular key role in transmission or spread is played by cats, which are the only domestic animals that serve as a final host. In cats that are primarily infected by cysts, a massive excretion of oocysts, which can last up to 20 days, occurs after a prepatency period of 3 to 10 days. The majority of bradyzoites released from ingested cysts usually remain in the small intestine. They initiate merogony and gametogeny in the intestinal epithelium, giving rise to the unsporulated oocysts. The remaining bradyzoites immediately penetrate the intestinal wall and reach the other internal organs via the lymphatic and blood pathways, resulting in extraintestinal development of the parasite. In contrast, when the cat is infected with oocysts, asexual reproduction must immediately take place in the extraintestinal organs. Some of the tachyzoites subsequently migrate into the intestinal wall and initiate development in epithelial cells until oocysts are formed. After a prepatency of 18 to 36 days, about 50 % of the cats excrete oocysts with the faeces.
The oocysts excreted by the cat are initially unsporulated and non-infectious. The generation of the infectious sporulated oocysts takes 1 to 5 days under conditions of sufficient oxygen supply, moisture, and temperature. Due to their very high resistance to environmental destruction, the sporulated oocysts pose a high risk of infection for humans and other animals. Once the intermediate host is infected, the bradyzoites or sporozoites immediately penetrate the intestinal wall after oral ingestion of the oocysts or cysts. This is followed by lymphohaematogenous spread into various organ systems, including mesenteric lymph nodes, liver, lungs and striated muscles. Two asexual multiplication phases are carried out in the organs. Intestinal development is absent.
Macroscopic and microscopic findings: Depending on the localization of the parasitic structures, macroscopic and histologic lesions can be found in several organs. The lungs, brain, and liver are most commonly affected by the changes. 4 The lungs show interstitial pneumonia, type II pneumocyte hyperplasia, and a necrotizing component associated with edema and hemorrhage. The liver shows mild hepatomegaly and clearly visible grey, white, or yellow foci. In histopathology of the liver, as in the other affected organs, necrosis is dominant. In the brain, T. gondii is found in most cases as a tachyzoite form in macrophages, glial cells, or neurons. Furthermore, it is possible to detect tissue cysts. In all affected organs, macrophages, lymphocytes and plasma cells are the dominant cell population of the inflammatory component. The formation of granulomas can occur. There may be a minor and variable neutrophilic component.
In a previously published case report of toxoplasmosis in a porcupine, microscopic changes were found in the liver, lungs, heart, and spleen, in agreement with the present case.6 Reported histopathologic findings in porcupines include necrotizing hepatitis, lymphohistiocytic and necrotizing myocarditis, lymphohistiocytic encephalitis, and lymphohistiocytic interstitial nephritis.6 Thin-walled tissue cysts with numerous bradyzoites, as well as tachyzoites, have been described within alveolar macrophages, free in the alveoli, between myocardial fibers, and within glomeruli and tubular epithelial cells.6
Diagnosis: T. gondii infection can be diagnosed by many different methods. For the selection of the most suitable diagnostic procedure, the immune status of the patient, the clinical signs, and the severity of the symptoms should be considered. The detection can be done indirectly using serology or by direct detection of the parasite antigen or DNA.10 The following techniques can be used: polymerase chain reaction (PCR; parasite detection), enzyme-linked immunosorbent assays (ELISAs; IgG/IgM/IgA detection; serology), indirect fluorescence antibody tests (IFATs; serology), comparative Western blotting (serology), Sabin-Feldman dye test (serology), and mouse bioassay. In veterinary medicine, histopathology and immunohistochemistry are of primary diagnostic importance. Furthermore, the diagnosis can be confirmed by electron microscopy.
Differential diagnosis: The genera Hammondia, Neospora and Besnoitia should be considered as etiologic differential diagnoses.3 Like the genus Toxoplasma, these belong to the family Toxoplasmatidae. A reliable differentiation of the oocysts of the named genera is not possible by means of histological examination alone.
Contributing Institution:
Institute of Veterinary Pathology
Faculty of Veterinary Medicine
Leipzig University
Leipzig, Germany
https://www.vetmed.uni-leipzig.de/institut-fuer-veterinaer-patholgie/
JPC Diagnosis:
Liver: Hepatitis, necrotizing, subacute, multifocal, random, mild to moderate, with intracytoplasmic apicomplexan zoites.
JPC Comment:
The contributor provides an excellent, thorough overview of toxoplasmosis, and rightly notes that Toxoplasma gondii infection has been documented in a wide range of mammalian hosts. Prevalence rates vary, but are throught to surpass 50% in dogs, rabbits, and sea otters; 60% in rats, mice, and birds; and 70% in humans, cats, bears, and deer.13
T. gondii has been extraordinarily successful by protozoal standards, and its successful transmission to many species worldwide is due, in part, to its ability to modify its hosts’
behavior for its own benefit.13 This “manipulation hypothesis” explains how parasites that are immature in the intermediate host ensure that they are eaten by the appropriate definitive host so the parasite can mature and complete its life cycle.13 The classic toxoplasmosis pairing is the cat, which serves as the definitive host in which gamteogeny occurs, and the mouse, in which shizogeny occurs, and this classic pair provides an illustrative example of the manipulation hypothesis. Infective oocysts, which develop in the feline host, are ingested by the wild rodent, where the parasites
undergo asexual reproduction followed by encystation in multiple tissues, including the brain.13 Because sexual maturation can be accomplished only in felines, there is strong evolutionary pressure for the parasite to develop mechanisms to ensure transmission from the mouse to the cat through predation.13
Researchers investigating the behaviors of T. gondii-infected mice found that the infected mice showed decreased learning capacity and memory compared to their uninfected counterparts. Because cats are more attracted to moving, exposed prey, investigators conducted a series of studies to determine if T. gondii infection increased activity levels. Researchers determined that infected mice were more active than their uninfected counterparts and spent more time in exposed or novel environments than control mice.13
Subsequent mouse and rat studies evaluated whether T. gondii infection affected rodents’ perception of cat predation risk as measured by their response to cat odor.13 Cat odor is well-known to elicit aversion behavior in laboratory rodents, even after hundreds of generations have been raised in the laboratory environment with no lived experience with feline predation.13 Results show that, while uninfected rodents show the expected aversive behaviors toward cat-treated areas, infected rodents exhibited not only a reduction in this aversive behavior, but also exhibited a significant, potentially suicidal preference for cat-treated areas.13
Convinced that T. gondii has behavior-modifying effects, researchers investigated whether treatment with antipsychotic drugs, many of which were known to inhibit T. gondii replication in cell cultures, could ameliorate the behavioral effects of T. gondii in rodents.13 Infected but untreated rodents demonstrated the same attraction to feline odors as seen previously; however, following treatment with schizophrenia drugs such as haloperiodol and valproic acid, such cat-seeking behaviors, as well as the numbers of neurons and glial cells immunohistochemically positive for T. gondii, were significantly reduced.13
The mechanisms by which T. gondii modifies rodent behavior are currently unknown, though neuromodulation has been suggested as the dominant mechanism. Studies attempting to determine the neurologic basis of anxiety often use the reaction of rodents to cats as a research model, and such studies have found that blocking certain receptors in the amygdala or providing serotonin antagonists causes rodents to display the same lack of aversion to cats that T. gondii-infected rodents display.13 Additional research has shown substantial differences in certain neurotransmitter levels in infected versus uninfected rodents, suggesting additional mechanisms by which T. gondii may be achieving its behavior modulatory effects.13 Additional research is needed to further elucidate the protozoal magic that causes normally avoidant rodents to run headlong into the jaws of a primary predator. Though interesting in its own right, understanding these mechanisms could have profound implications for human behavior given the high levels of suspected T. gondii infection in the human population.
The moderator emphasized the need for molecular diagnostics when attempting to differentiate among the various apicomplexans, many of which look identical on H&E examination. Participants appreciated this straight-forward example of hepatic toxoplasmosis, which sparked a discussion of how an organism such as T. gondii, which has no known virulence factors, can cause such significant necrosis.
References:
- Aguirre AA, Longcore T, Barbieri M, et al. The One Health approach to toxoplasmosis: epidemiology, control, and prevention strategies. EcoHealth. 2019;16(2):378-90.
- Denk D, Neck S de, Khaliq S, Stidworthy MF. Toxoplasmosis in zoo animals: A retrospective pathology review of 126 cases. Animals. 2022;12(5).
- Deplazes P, Eckert J, Samson-Himmelstjerna G von, Zahner H. Lehrbuch der Parasitologie für die Tiermedizin. 3. Auflage. Stuttgart: Enke Verlag; 2013; 87-94.
- Dubey JP, Carpenter JL. Histologically confirmed clinical toxoplasmosis in cats: 100 cases (1952-1990). J Am Vet Med Assoc. 1993;203(11):1556-1566.
- Control of intestinal protozoa in dogs and cats. ESCCAP Recommendation No. 6. https://www.esccap.de/empfehlung/protozoen/. Updated May 30, 2022. Accessed May 30, 2022.
- Fayyad A, Kummerfeld M, Davina I, et al. Fatal systemic Toxoplasma gondii infection in a red squirrel (Sciurus vulgaris), a swinhoe's striped squirrel (Tamiops swinhoei) and a New World porcupine (Erethizontidae sp.). J Comp Pathol. 2016;154 (2-3):263-267.
- Fuglewicz AJ, Piotrowski P, Stodolak A. Relationship between toxoplasmosis and schizophrenia: A review. Adv Clin Exp Med. 2017;26(6):1031-1036.
- Halonen SK, Weiss LM. Toxoplasmosis. Handb Clin Neurol. 2013;114:125-145.
- Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8(10):634-40.
- Robert-Gangneux F, Dardé M-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012; 25(2):264-296.
- Schnieder T, Boch J, eds. Veterinär-medizinische Parasitologie. 6. Aufl. Stuttgart: Parey; 2006; 144-148, 298, 366-367, 428-432.
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12-13): 1217-1258.
- Webster JP. The effect of Toxoplasma gondii on animal behavior; playing cat and mouse. Schizophr Bull. 2007;33(3):752-756.