Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 4
September 20th, 2017
CASE IV: CASE #2 (JPC 4101755).
Signalment: 11 year old, castrated male, French bulldog, (Canis lupus familiaris).
History: A veterinarian found a mass at the base of the heart and tried to treat with radiation therapy. Clinically, the dog had severe coughing because of tracheal compression from the mass. A tracheostomy was performed since the larynx had collapsed. The dog was found dead by his owner 17 days after starting radiation therapy.
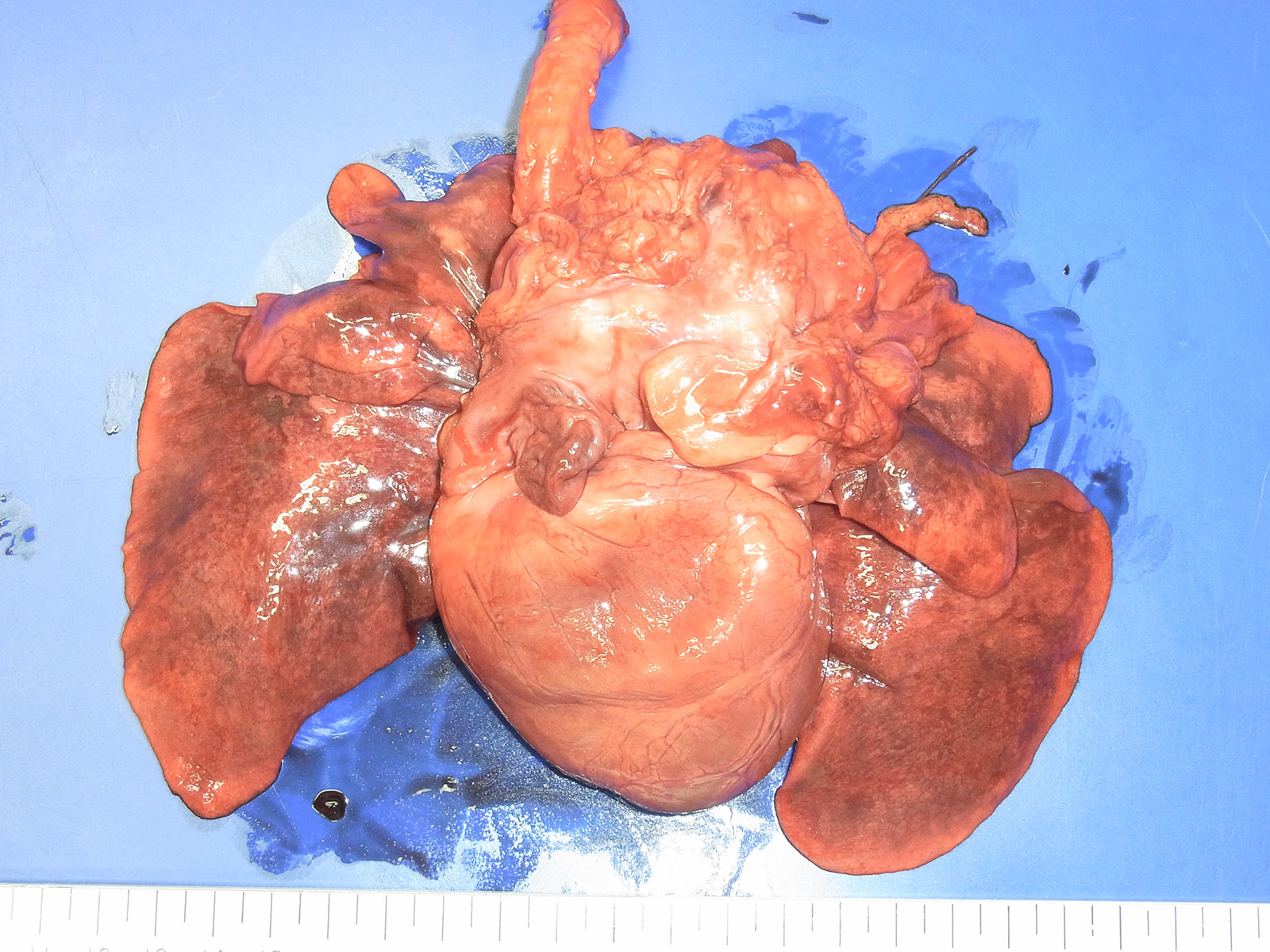
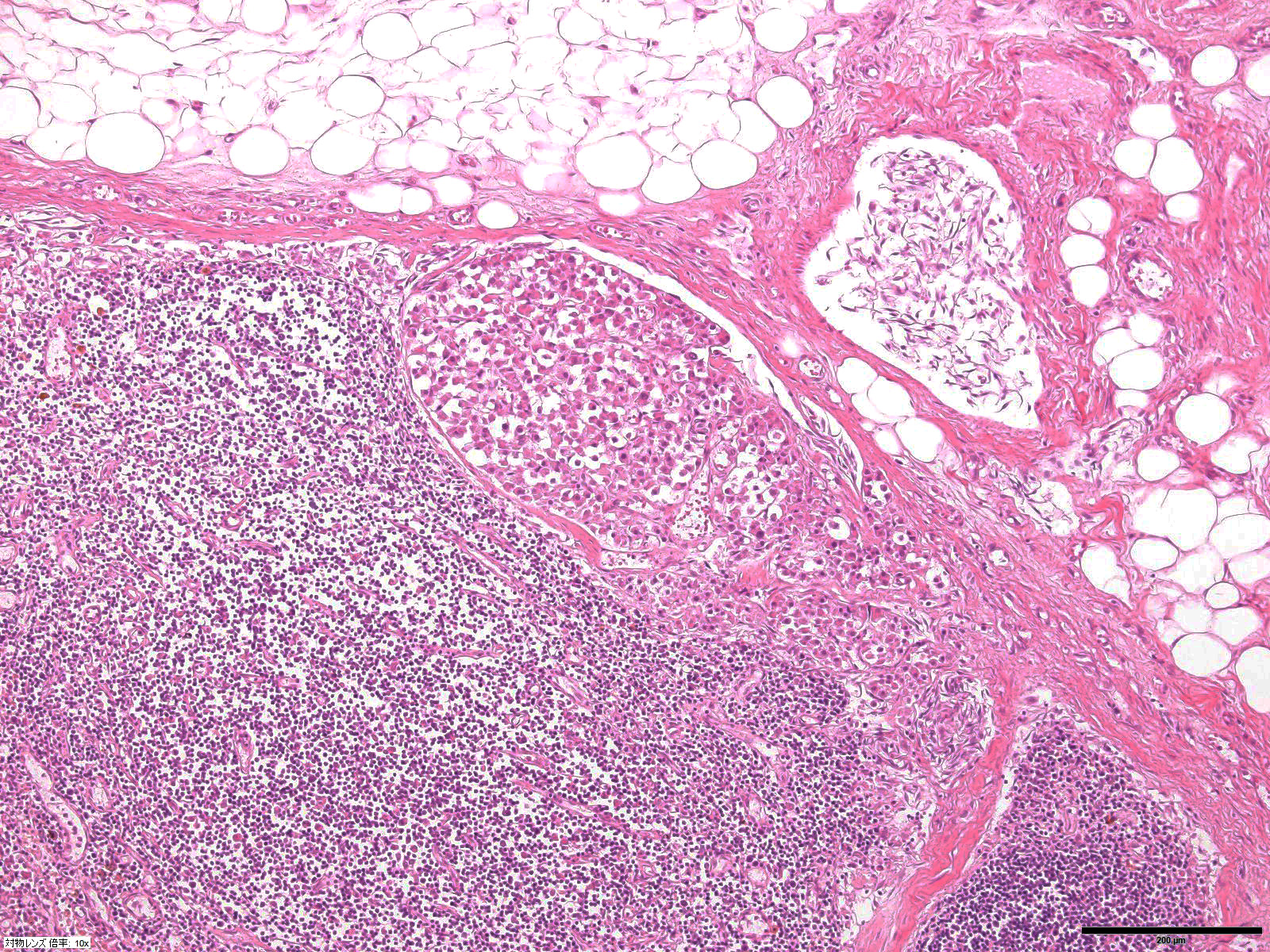
Gross Pathology: Necropsy was done only for the organs in the thoracic cavity. A 7x7x5 cm, reddish-tan to grayish-white, multi-nodular mass was found at the base of the heart involving the aorta, vena cava and trachea. The lungs were mildly edematous and appeared reddish-tan in color.
Laboratory results: None provided.
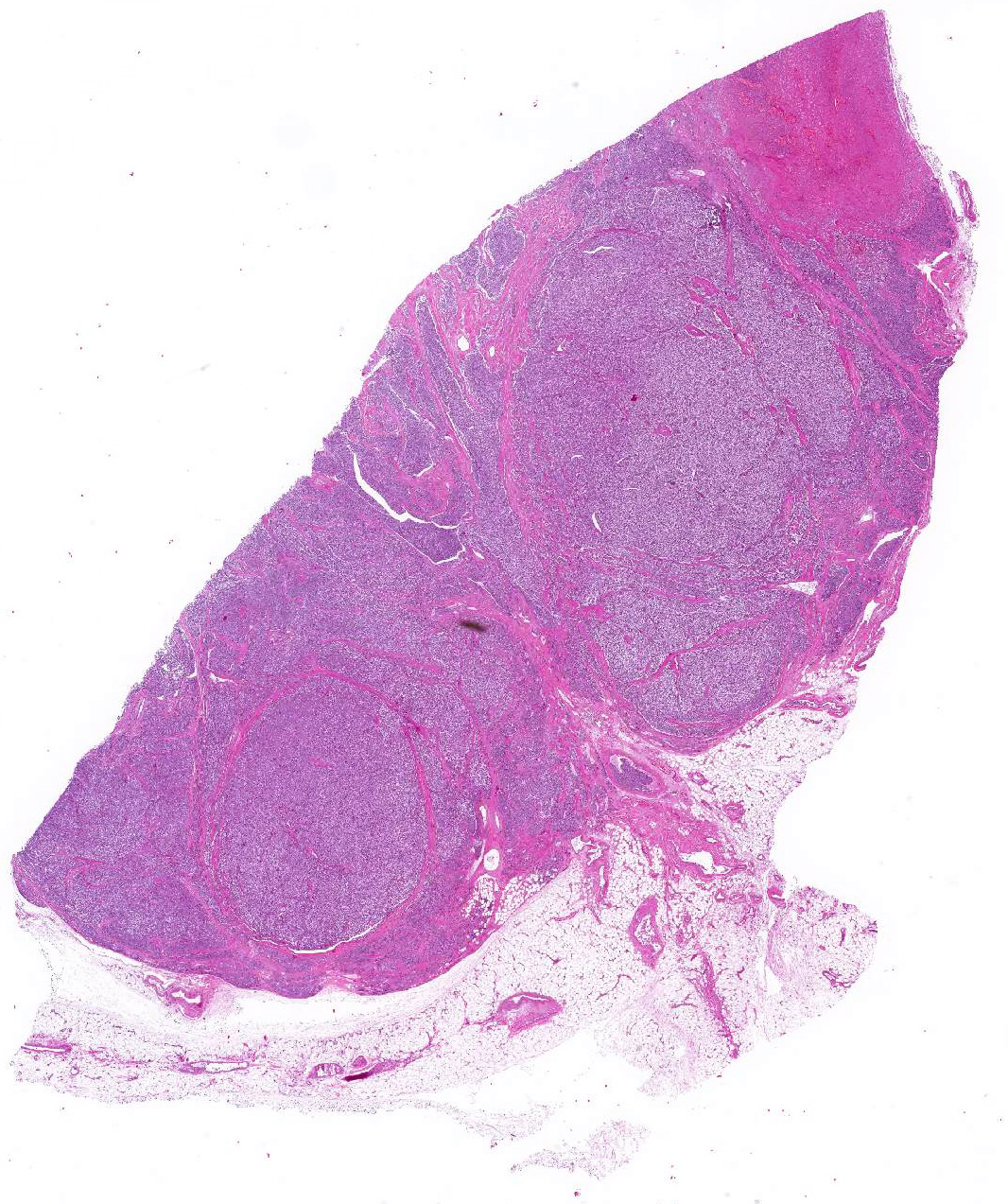
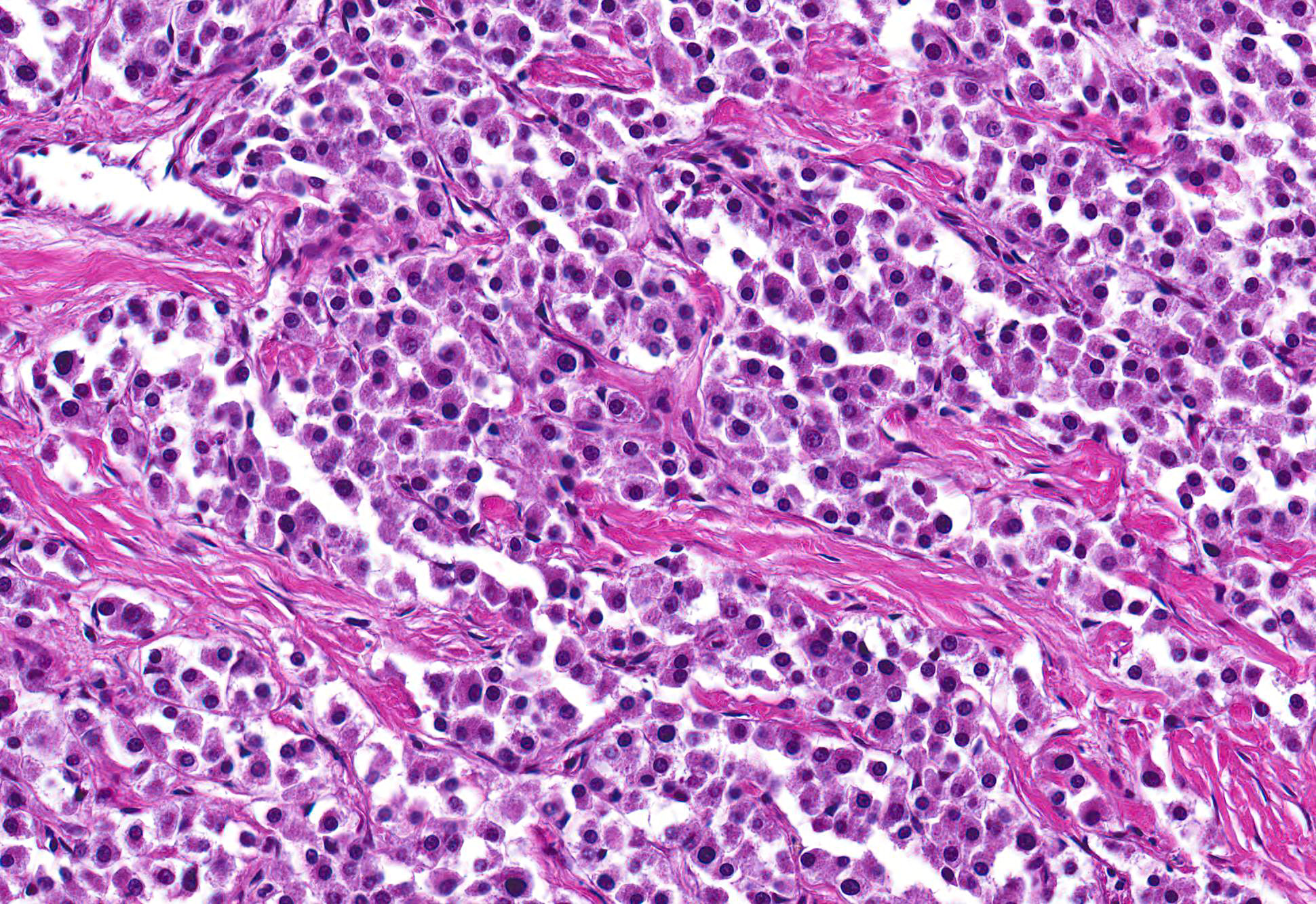
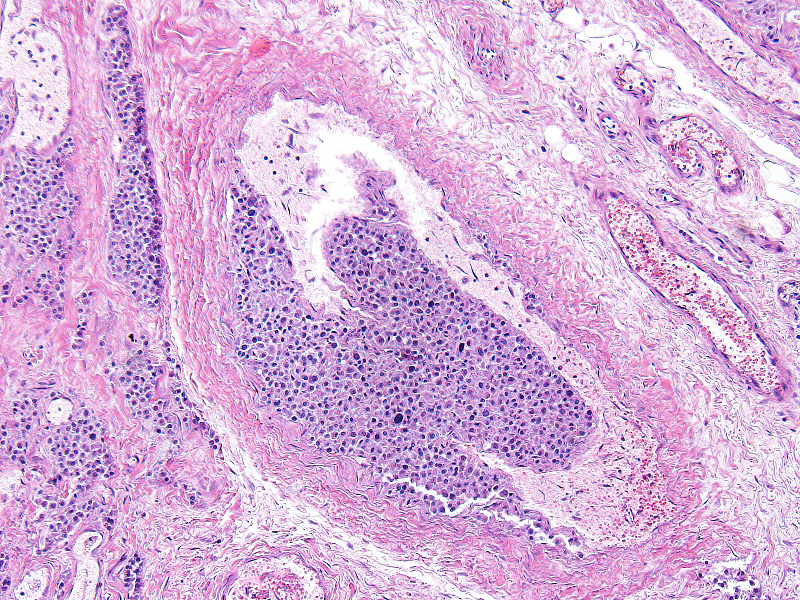
Microscopic Description: Heart: The mass is poorly demarcated and invades the right atrium wall. The neoplastic cells are polygonal arranged in cords on a moderate fibrovascular stroma. The nuclei are round to ovoid, with rare distinct nucleoli, and moderate anisokaryosis and anisocytosis. Mitoses are rare. The cytoplasm is plump with fine eosinophilic granules. Atypical neoplastic cells with giant nuclei are occasionally found especially in the center of the mass. There are multifocal areas of necrosis, and vascular invasion is frequently seen with clusters of neoplastic cells in blood vessels in the less affected area of the heart.
Contributors Morphologic Diagnosis: Heart: Aortic body carcinoma.
Contributors Comment: Aortic body tumor is a type of paraganglioma (chemodectoma) which is derived from cells of the neural crest. In dogs, paragangliomas are predominantly derived from the aortic or the carotid body. Aortic body tumors are more frequent than carotid body tumors in animals, whereas it is opposite in humans. Paragangliomas occur most frequently in dogs with lower incidence in cats and cattle. Brachycephalic breeds such as the Boxer and Boston terrier are highly predisposed, which implies that some genetic predisposition that is aggravated by chronic hypoxia seems to be cause of this tumor. Paraganglioma does not cause functional clinical signs, but it can compress the trachea, aorta and vena cava resulting in cardiac decompensation (hydropericardium, hydrothorax, cyanosis, ascites, edema, and passive congestion of the liver) and/or dyspnea, coughing, or vomiting.7
Aortic body tumors are usually benign but malignant tumors can also occur1,8. Aortic body carcinomas can infiltrate the wall of the pulmonary artery to form papillary projections into the lumen or invade the wall of the left atria. Aortic body carcinomas can metastasize to many organs such as the lung, liver, myocardium, kidney, lymph nodes and adrenal cortex7. A recent report has indicated that 9 out of the 13 dog (69%) cases showed metastasis to other organs9. The authors compared the characteristics of metastatic and non-metastatic aortic body carcinomas and demonstrated that metastasis is correlated with high tumor weight to body weight ratio (g/kg). However, no significant difference was found in malignant features of neoplastic cells such as pleomorphism and presence of giant cells. They concluded that those tumors are generally malignant or potentially malignant. In our case, vascular invasion of neoplastic cells and metastasis to a hilar lymph node were found. Unfortunately, we were prevented from investigating organs outside of the thoracic cavity at the request of the owner.
In humans, genetic mutations of succinate dehydrogenase complex subunit D (SDHD) in familial paraganglioma were first identified in 20002. SDHD protein is one of the subunits consisting succinate dehydrogenase (Complex II of the respiratory chain) integrated in the inner mitochondrial membrane. SDHD forms dimer with SDHC, another subunit of Complex II. The dimer can be bound to ubiquinone and water during electron transport at Complex II. Genetic mutations of SDHD can decrease the enzymatic activity of Complex II and lead to cellular hypoxia. Although the exact mechanism of tumorigenesis by SDHD mutations is still unclear, hypoxia due to decreasing Complex II activity may be associated with tumorigenesis. Indeed, people living at higher altitudes (e.g. Andes peoples), are subject to paraganglioma and hypoxia-inducible factors (HIF) affect several biological events related to tumorigenesis such as cell proliferation, metabolism and angiogenesis. Mutations of other SDH protein composing Complex II (SDHA, SDHB, SDHC) are also associated with paraganglioma5,7. Specifically, SDHB mutation frequently results in metastatic paraganglioma, whereas SDHD mutation is usually related to benign paraganglioma in the head and neck3. In dogs, a study indicated genetic mutations of SDHD and SDHB in some chemodectomas and pheochromocytomas4. Canine chemodectomas have the potential to be a model for human paraganglioma but further research is required.
JPC Diagnosis: Fibroadipose tissue: Neuroendocrine tumor, French bulldog, canine.
Conference Comment: This case provided a beautiful representation of a neuroendocrine tumor in a brachycephalic dog. In the slides provided, there was no myocardium present, and attendees were unable to be more definitive in their diagnoses than neuroendocrine tumor.
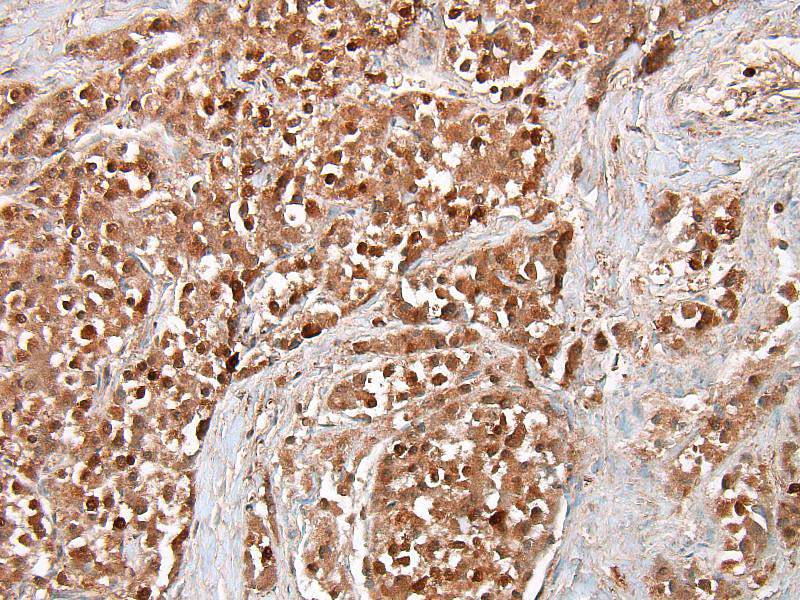
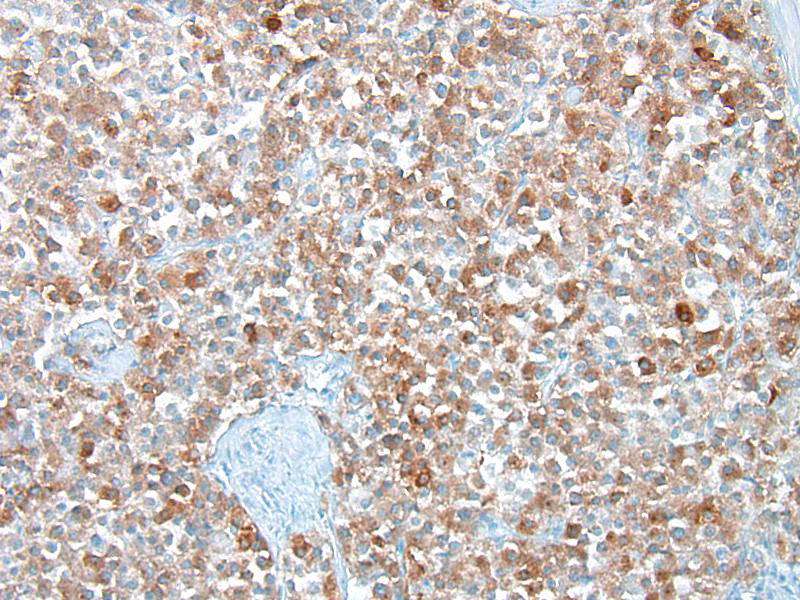
Conference participants discussed several stains that could be used to identify this as a neoplasm of neuroendocrine origin. Secretory granules of neuroendocrine cells can be identified with chromogranin A, neuron-specific enolase, synaptophysin, and S100. Churukian-Schenk, a silver-bassed, histochemical stain may also be used to identify granules. Ultrastructurally, secretory granules appear electron-dense and membrane-limited. There are also stellate or sustentacular cells with long cytoplasmic processes present in-between neoplastic cells. These cells are theorized to provide support to the chemoreceptor cells. Malignant tumors may have decreased secretory granules and sustentacular cells, and some stains (chromogranin A) could potentially be negative.7
Chemoreceptor organs are located at the base of the heart (aortic body) and in the neck (carotid body) and function as sensors of variations in blood carbon dioxide content, pH, and oxygen tension and help to regulate respiration (through parasympathetic nerves) and circulation (through sympathetic nerves) based on detected changes. These organs are small and composed of chemoreceptor cells and sustentacular cells on a fine collagen and reticular fiber stroma. Chemoreceptor cells are of neural crest origin and have intracytoplasmic secretory granules that contain vasoactive factors, dopamine, norepinephrine, enkephalin peptides, and adrenomedullin. In addition to the carotid and aortic bodies, chemoreceptors are located in the nodose ganglion (vagus nerve), ciliary ganglion (orbit), pancreas, below the middle ear on the internal jugular vein, and the glomus jugulare (recurrent branch of the glossopharyngeal nerve).7
Participants were encouraged to read a recent article that outlines the findings in 13 cases of canine aortic body tumors in which 9 dogs had metastases and 4 did not. A recent publication on aortic body tumors in 13 dogs9 identified tradition features of malignancy in these tumors, including pleomorphism, anisokaryosis and anisocytosis, mononuclear giant cells, and local tissue and vascular invasion, but none correlated with metastasis. Hence, these neoplasms should be all considered as potentially malignant.
Laboratory of Comparative Pathology
Department of Veterinary Clinical Sciences
Graduate School of Veterinary Medicine
Hokkaido University
https://www.vetmed.hokudai.ac.jp/organization/comp-pathol/e/index.html
References:
1. Aupperle H, März I, Ellenberger C, Buschatz S, Reischauer A, Schoon HA. Primary and secondary heart tumours in dogs and cats. J Comp Pathol. 2007;136:18-26.
2. Baysal BE, Ferrell RE, Willett-Brozick JE, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848-851.
3. Favier J, Brière JJ, Strompf L, et al. Hereditary paraganglioma/pheochromocytoma and inherited succinate dehydrogenase deficiency. Horm Res. 2005;63:171-179.
4. Holt DE, Henthorn P, Howell VM, Robinson BG, Benn DE. Succinate dehydrogenase subunit D and succinate dehydrogenase subunit B mutation analysis in canine phaeochromocytoma and paraganglioma. J Comp Pathol. 2014;151:25-34.
5. Kirmani S, Young WF. Hereditary Paraganglioma-Pheochromocytoma Syndromes. 2008 May 21 [Updated 2014 Nov 6]. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017.
6. Rijken JA, Niemeijer ND, Jonker MA, et al. The Penetrance of paraganglioma and pheochromocytoma in SDHB germline mutation carriers. Clin Genet. 2017; [Epub ahead of print] doi: 10.1111/cge.13055.
7. Rosol TJ, Meuten DJ. Tumors of the endocrine glands. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Oxford, UK: John Wiley & Sons, Inc.; 2017:828-833.
8. Treggiari E, Pedro B, Dukes-McEwan J, Gelzer AR, Blackwood L. A descriptive review of cardiac tumours in dogs and cats. Vet Comp Oncol. 2017;15:273-288.
9. Yamamoto S, Fukushima R, Hirakawa A, Abe M, Kobayashi M, Machida N. Histopathological and immunohistochemical evaluation of malignant potential in canine aortic body tumours. J Comp Pathol. 2013;149:182-191.