Signalment:
Age unspecified
female Columbia X Rambouillet ewe (
Ovis aries).A
flock of 7500 ewes were grouped in mobs of 1500. Over the course of the 2016
lambing season, 1200 ewes, ranging in age from 2 8 years aborted. Abortions
continued even after feeding tetracycline pellets. At the end of the lambing
season, older aborted ewes were culled and younger recovered ewes were mixed
with ewe lambs as a vaccination strategy.
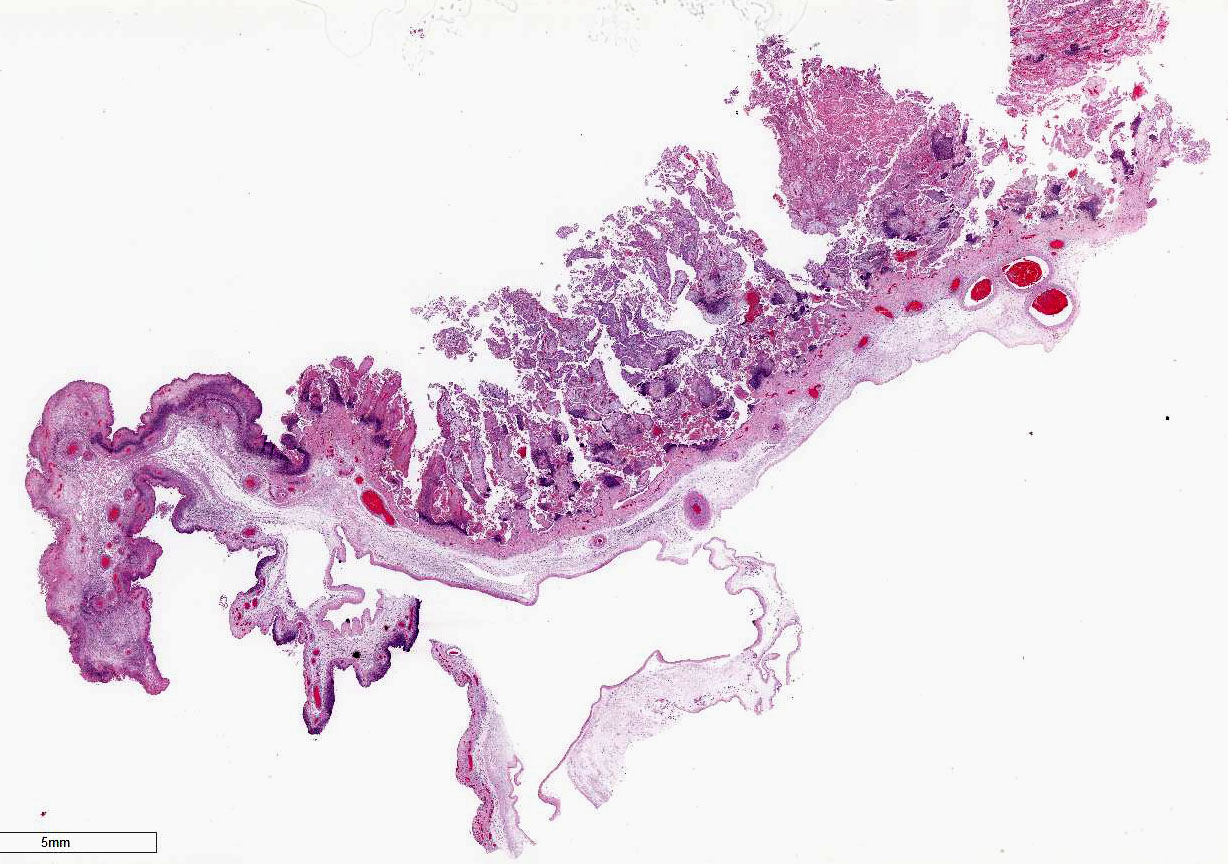
Gross Description:
Three fetuses
and placentas were markedly autolyzed and had no significant gross lesions. A
fourth placenta was in good to fair post-mortem condition. That placenta had
inter-cotyledonary edema and multifocal tan-grey discoloration of cotyledons.
Histopathologic Description:
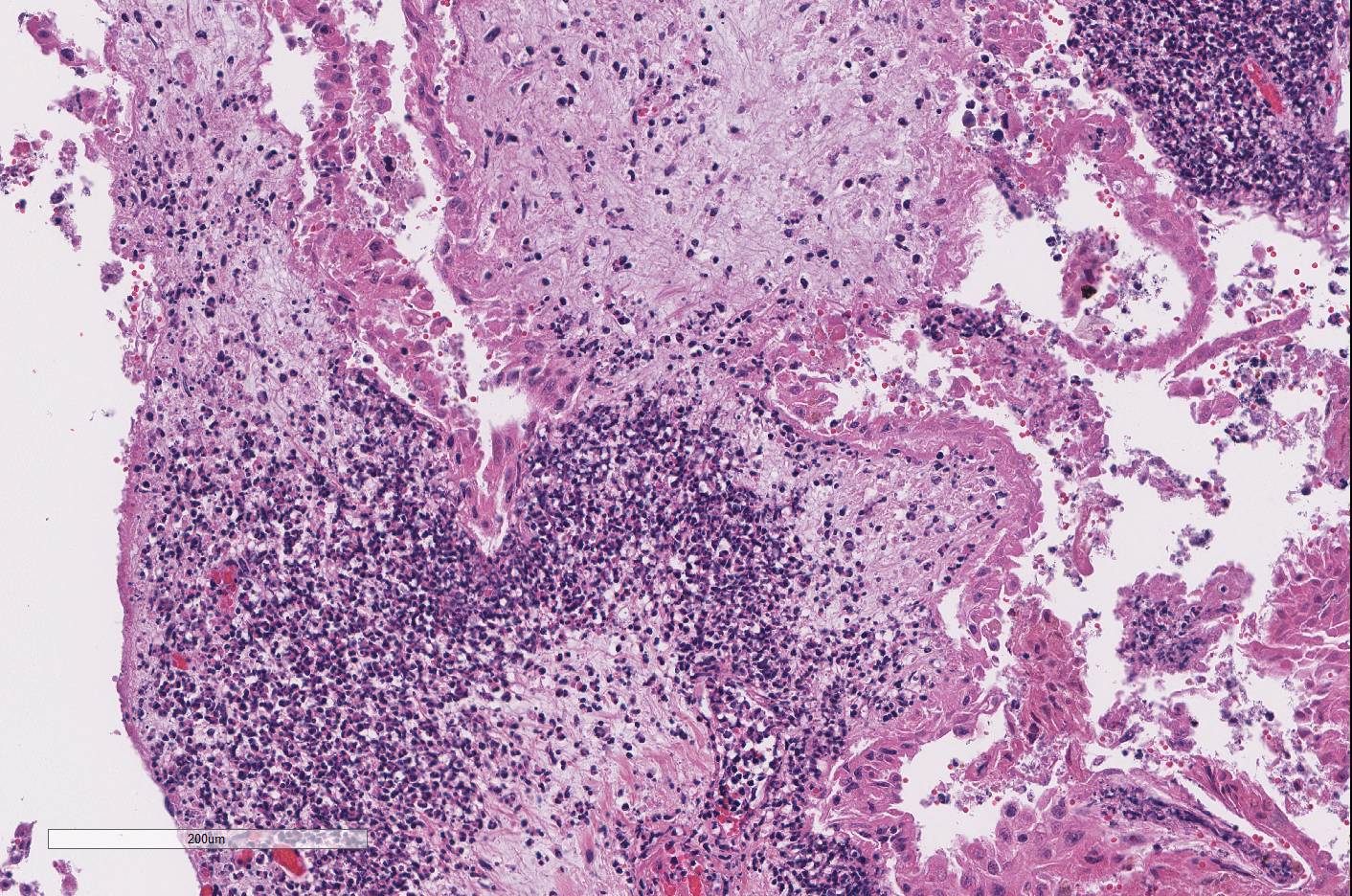
Both
cotyledonary and intercotyledonary areas were characterized by necrosis of
tropho-blastic and intercoteledonary epithelium, with hypereosinophilic
shrunken cytoplasm and karyorrhectic or karyolytic nuclei. Foci of necrosis
were expanded by necrotic cellular debris and large numbers of degenerate
neutrophils within the immediate underlying chorioallantoic connective tissue.
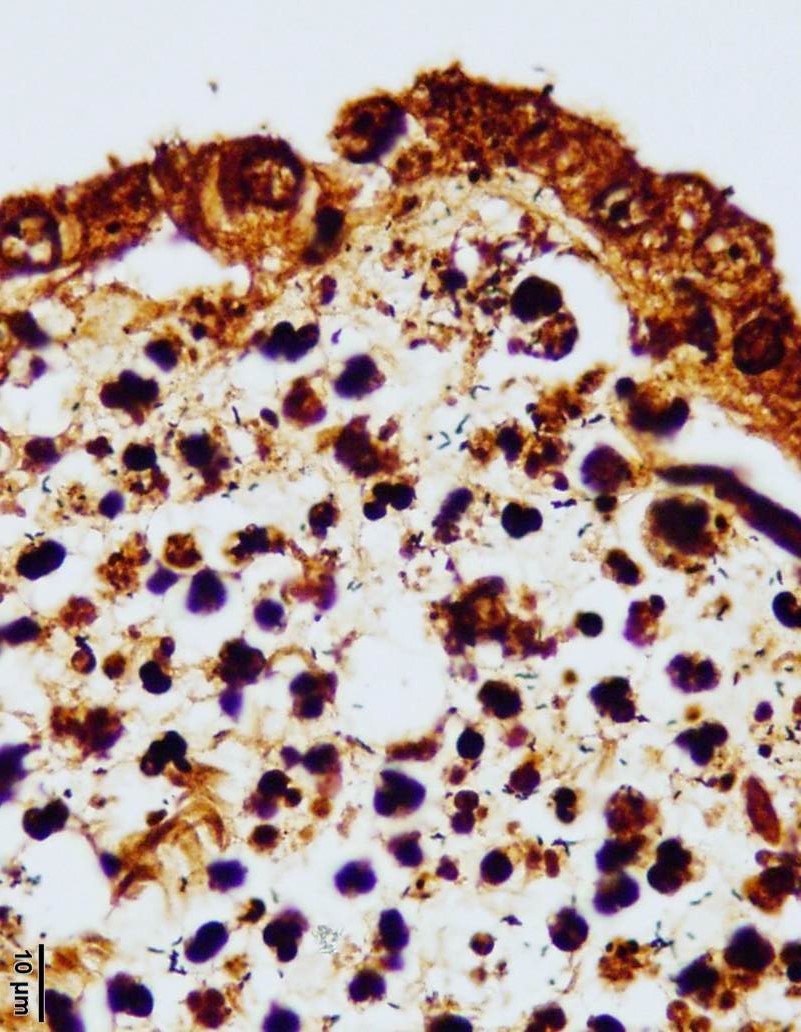
Several areas of necrosis are associated with many clustered bacilli. Adjacent
tropho-blasts often contain small intracytoplasmic bacilli. Rare trophoblasts
contain, intra-cytoplasmic, 2 μm diameter, basophilic material. Scattered
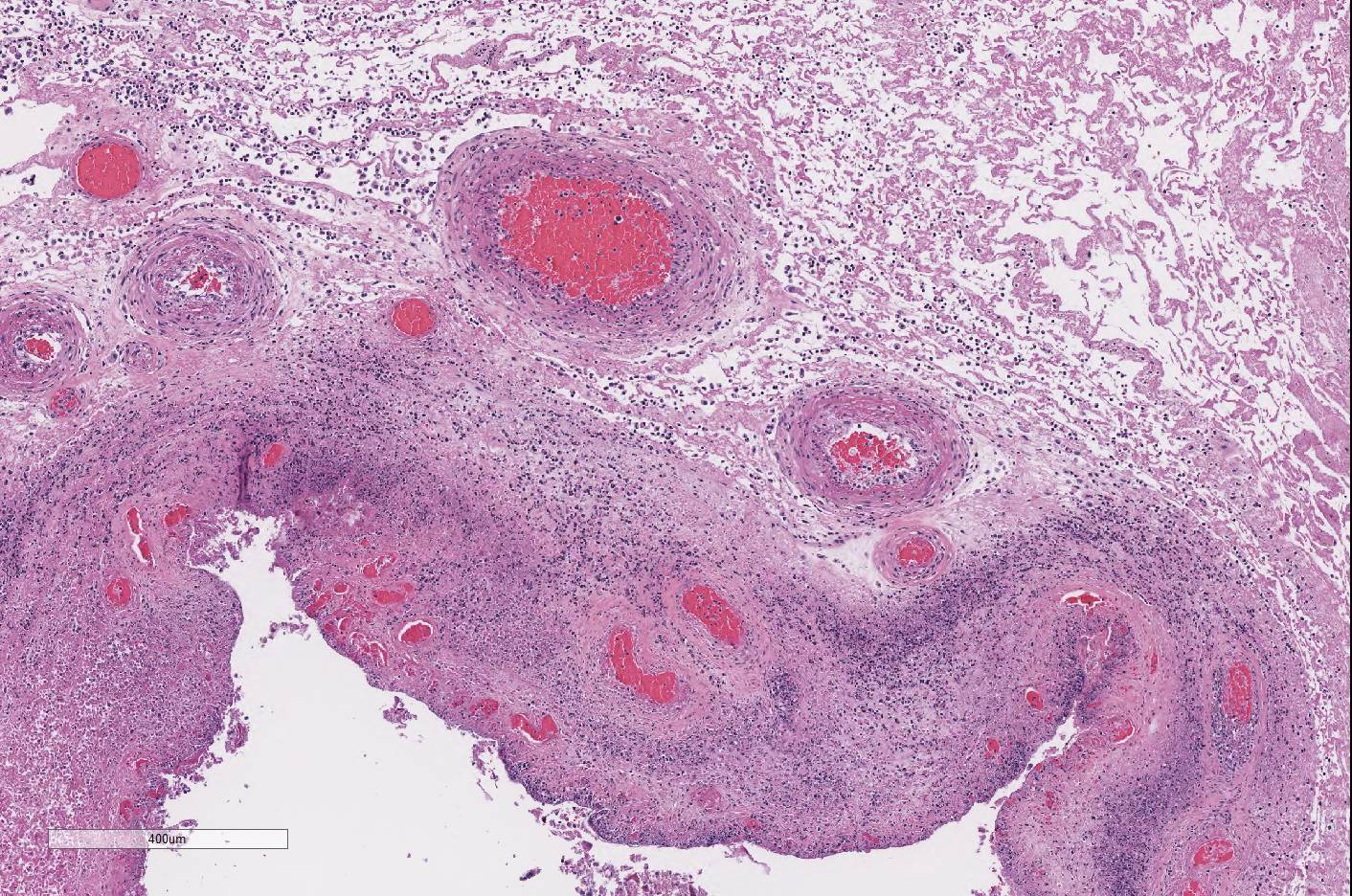
throughout the chorioallantois, many blood vessels are surrounded by
neutrophils and a few vessels are partially occluded by large aggregates of
fibrin and neutrophils. Rare small vessels are lined by necrotic endothelial
cells with separation of the wall by neutrophils and fibrin (fibrinoid
necrosis). Diffusely the chorioallantoic connective tissue is expanded by edema
and all vessels are markedly congested.
Similar lesions
were seen in placentas from the other fetuses submitted. No significant
lesions were identified in any of the fetal tissues.
Morphologic Diagnosis:
Placentitis, necrosuppurative, cotyledonary and
intercotyledonary, diffuse, severe, with necrotizing vasculitis and bacteria.
Lab Results:
Numerous
Campylobacter jejuni were isolated from one of four placentas. Other
tests performed with negative results included FA for
Leptospira interogans,
ELISA for
Chlamydophila sp. and PCR for pestivirus. Selenium level in
one of three livers was marginal. Immunohistochemistry for
Coxiella
burnetii on fixed placenta was negative.
In two previous
submissions from the same farm,
C. jejuni was isolated from fetal tissue
pools, stomach contents or placentas from four additional aborted fetuses.
Condition:
Necrosupporative placentitis/Campylobacter jejuni
Contributor Comment:
Campylobacter
jejuni is one of three species in the genus causing reproductive and enteric disease
in a variety of animal species and in humans.
8 C. fetus subsp.
venerealis primarily causes infertility and abortion in cattle, whereas
C.
fetus subsp fetus and
C. jejuni are important causes of abortion in
small ruminants and occasionally cattle.
C. jejuni is an important cause
of food-borne illness in people and has become an increasingly important cause
of late term abortions in small ruminants.
4 Abortions have also been
documented in humans and in dogs.
6 In sheep, infection by either
C.
fetus fetus or
C. jejuni causes late term abortion, still birth or
weak lambs. Transmission is by the oral route. Placentas are not retained and
often have gross lesions of intercoteledonary edema and cotyledonary necrosis.
Aborted fetuses may have characteristic gross lesions of necrotizing hepatitis
and histologic lesions of suppurative bronchopneumonia (neither present in this
case). Occasionally ewes become ill and die due to endometritis.
8
Campylobacter is reportedly
the most common cause of abortion in sheep and
C. jejuni is now the most
common species to cause abortion in sheep flocks.
4 Recently, a
single clone of
C. jejuni (SA) has been shown experimentally to cause
abortion in sheep
9 and it is genetically similar to clones causing
gastroenteritis in people.
5 In addition, most isolates of
C.
jejuni from sheep abortions in the United State (including the one in this
case) are highly resistant to tetra-cyclines, the only approved drug for
treating infection in sheep.
4 In contrast, isolates from the United
Kingdom are susceptible to tetracyclines
9, suggesting common
treatment of sheep abortions with teracyclines in this country may have led to
the emergence of the resistant clone. Drug resistance and links to human
enteric disease indicate that owners, veterinarians, and laboratory personnel
should be cautioned about zoonotic potential when handling suspect fetal
tissues.
Other
important differential diagnoses for placentitis in sheep and goats include
Brucella
ovis,
Brucella mellitensis,
Toxoplasma gondii,
Chlamydophila
abortus and
Coxiella burnetii.
8 The most common
manifestation of ovine brucellosis in the US is epididymitis in rams; abortion
with placentitis is less common.
Toxoplasma gondii should have
characteristic gross and histologic lesions, with the presence of organisms
within the placental lesions, but can also be ruled out by PCR. The three main
differentials, in this case, are
C. jejuni,
C. abortus and
C.
burnetii due to the presence of intracellular bacteria on H&E. The
bacteria were not positive with the Gimenez stain, making diagnosis of the two
latter organisms less likely.
C. abortus was eliminated by Ag ELISA on
the placenta and
C. burnetii by immunohistochemistry. Because of the
high likelihood of encountering zoonotic agents associated with small ruminant
abortions, examination of all sheep and goat abortuses, especially if
accompanied by fetal membranes, should be performed in a biosafety cabinet.
JPC Diagnosis:
Placenta,
cotyledonary and intercodyledonary: Placentitis, necrosuppurative, diffuse,
severe with necrotizing vasculitis.
Conference Comment:
The contributor provides a concise summary of abortion in small ruminants
caused by a
Campylobacter spp as well as other important differential
diagnoses for abortion in these animals. Despite some minor slide variability,
conference participants
unanimously noted the
outstanding preservation and high quality of the section of placenta in this
case.
Prior to discussing the case, the conference moderator spent
some time reviewing placentation in ruminants. The different components of the
placenta were discussed and participants identified individual layers and their
orientation within the tissue section. All ruminants have cotyledonary
villous epitheliochorial nondeciduate placentation. The placenta is comprised
of the maternal endometrium and the fetus derived fused chorioallantoic
membranes (CAM). Because ruminant placentas are nondeciduate, the maternal
endometrium and fetal CAM are in contact but they do not fuse. In
addition, in cotyledonary placentation, there are multiple areas where the CAM
villi insert into pockets or crypts in the area of the endometrium known as the
placentome, which is a combination of the fetal cotyledon and maternal
caruncle. Specific to small ruminants, the caruncles have lost their
epithelium, leaving five tissue layers which separate maternal and fetal blood:
endothelium, connective tissue, epithelium of the CAM, and endothelium and
connective tissue of the endometrium.1
Conference participants noted that, in this case, there
are multifocal brown globular pigment present in the maternal side of the
placenta in the subchorial area where hematoma and hemophagocytosis are often
most prominent and a normal finding. Additionally, thrombosis within the
chorionic plate , present in some slides in this case,
is also a normal finding in the post-partum placenta. However, if there is
similar brown staining material on the CAM, it could be indicative of meconium
deposition, which is a result of pre-parturient fetal stress. Meconium staining
was not seen by conference participants in this case.
Transmission of Campylobacter spp. often occurs via
fecal-oral route most commonly through contamination of water supplies.8
The organism is a common commensal bacterium in the intestinal tract of cattle,
sheep, and swine as well as dogs, cats, and rodents. When taken in orally in
susceptible animals, there is a transient bacteremia. The bacteria are then
localized to the gut and bile. In pregnant ewes, the bacteria localize to the
uterus via the Surface (S)-layer protein, which is thought to allow the
bacteria to colonize and translocate from the uterus to the placenta and
subsequent abortion in about 25% of cases.8
Characteristic findings of campylo-bacteriosis are edematous
intercotyledonary areas and friable yellow cotyledons with necrotizing and
suppurative placentitis and vasculitis most severe in chorionic villi. There
will often be large dense Gram-negative bacterial emboli within chorionic
capillaries, although that was not a prominent feature in this case.8
However, numerous Campylobacter spiral organisms are present throughout
the tissue and easily visualized on the Warthin-Starry silver stain. Many
conference participants noted intra-cellular bacilli within trophoblasts on the
H&E. In the fetus, there will typically be yellow hepatic foci with
targetoid depressed red centers (necrotizing hepatitis) and fibrinous
peritonitis.8
References:
1. Bacha
WJ, Bacha LM. Color Atlas of Veterinary Histology. 3rd
ed. Baltimore, MD: Lippincott Williams & Wilkins; 2012:243-260.
2. Headstrom OR,
Sonn RJ, Lassen ED, et al. Pathology of Campylobacter jejuni abortion
is sheep. Vet Pathol. 1987; 24:419-426.
3. Hazlett MJ, McDowall R, DeLay J, et al. A prospective
study of sheep and goat abortion using real-time polymerase chain reaction and
cut point estimation shows Coxiella burnetii and Chlamydophila
abortus infection concurrently with other major pathogens. J Vet
Diagn Invest. 2013; 25(3):359-368.
4. Sahin O, Plummer
PJ, Jordan DM, et al. Emergence of a tetracycline-resistant Campylobacter
jejuni clone associated with outbreaks of ovine abortion in the United
States. J Clin Micro. 2008; 46:1663-1671.
5. Sahin O,
Fitzgerald F, Stroika S, et al. Molecular evidence for zoonotic transmission of
an emergent, highly pathogenic Campylobacter jejuni clone in the United
States. J Clin Micro. 2012; 50:680-687.
6. Sahin O,
Burrough ER, Pavlovic N, et al. Campylobacter jejuni as a cause of
canine abortions in the United States. J Vet Diag Invest. 2014;
26:699-704.
7. Sanad YM, Jung
K, Kashoma I, et al. Insights into potential pathogenesis mechanisms associated
with Campylobacter jejuni-induced abortions in ewes. BMC Vet Res.
2014; 10:274-287.
8. Schlafer DH and
Foster RA. Diseases of the gravid uterus, placenta and fetus In: Maxie MG, ed.
Jubb Kennedy and Palmer's Pathology of Domestic Animals. Vol 3.
6th ed. Philadelphia, PA: Elsevier Saunders; 2016:407-408.
9. Wu Z, Sippy R,
Sahin O, et al. Genetic diversity and antimicrobial susceptibility of Campylobacter
jejuni isolates associated with sheep abortion in the United States and
Great Britain. J Clin Micro. 2014; 52:1853-1861.