Signalment:
The hamsters routinely developed chronic dermatitis, most severe over the shoulders and dorsal neck. This is the injection location of the immunizations that were given 4-5 times over a 2-4 month period. All hamsters were purchased from the same vendor and often arrived with a history of receiving ivermectin treatment for demodicosis. Treatment for the chronic dermatitis over the cervical dorsal area consisted of triple antibiotic ointment (Vetropolycin-�) applied three times per week topically.
Gross Description:
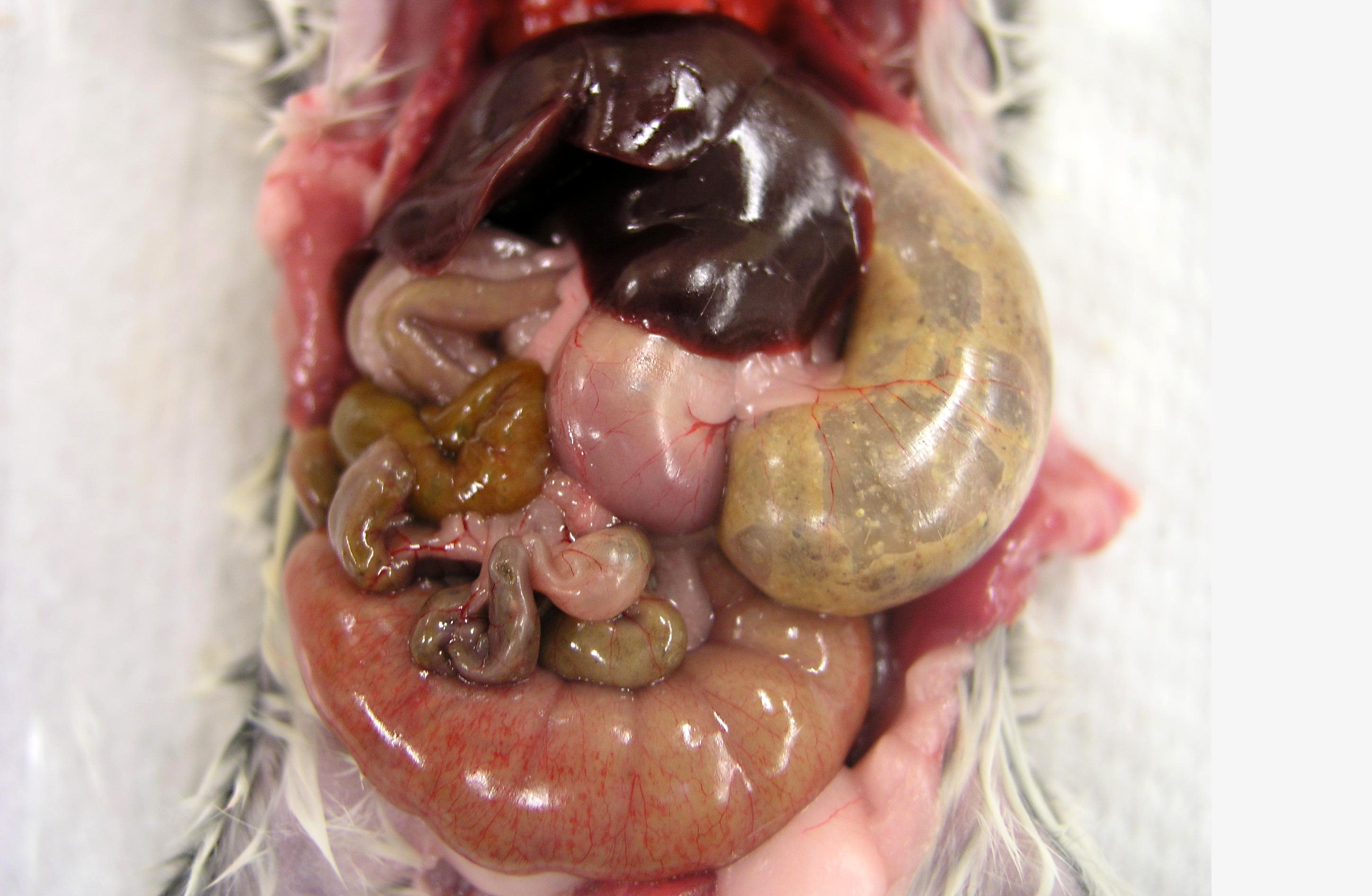
All of the hamsters had formed fecal pellets in the rectum and stomachs distended with food. The cecum and large intestine contained pasty ingesta. Several hamsters presented with congested ceca and proximal colons, characterized by serosal erythema and petechia. No other gross abnormalities were detected.Â
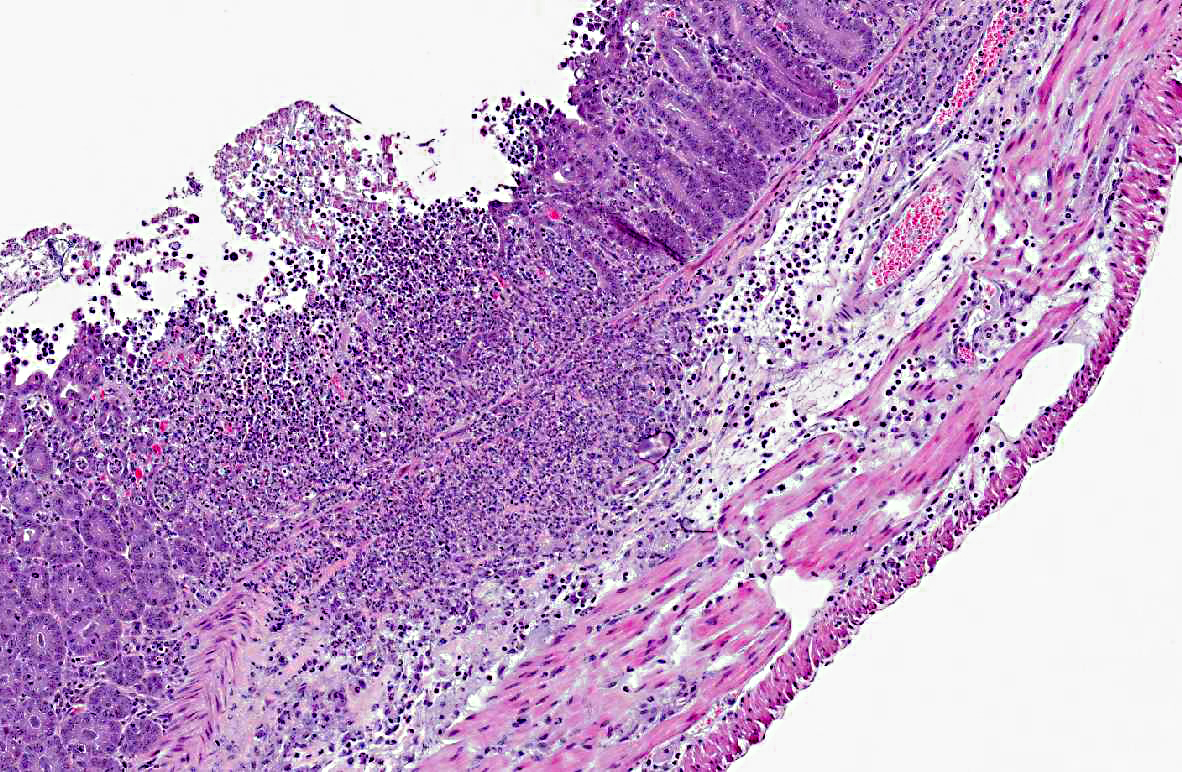
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Overgrowth of C. difficile occurs following the disturbance of the normal Bacteroides and Lactobacillus flora.(5) Clindamycin and ampicillin antibiotics are reported to directly enhance C. difficile colonization by increased expression of adherence factors beneficial for C. difficile colonization and adherence.3 Typhlitis and enterotoxemia develop secondary to the elaboration of toxins A and B, responsible for the tissue damage and influence of the immune response, resulting in watery or hemorrhagic diarrhea, dehydration and death within 2-10 days.(5) Two A exotoxins, an enterotoxin, and the cytotoxin B are produced by C. difficile. Epithelial tissue damage from the toxins is likely following the glycosylation and inactivation of Ras GTPases, disabling signaling pathways.(4) Toxins also glycosylate Rho, which regulates the actin cytoskeleton, resulting in the opening of tight junctions, and eventual cell apoptosis.(4) The toxins also cause release of proinflammatory mediators, attracting neutrophils, and activate secretion stimulated by the enteric nervous system.(4) Demonstration of the toxins is necessary for interpretation of the diagnostic significance of C. difficile.
This case is similar to a recently reported fatal clostridial typhlitis of a Syrian hamster treated with topical antibiotic ointment, containing polymixin B, neomycin and bacitracin.(1) That case surmised that self-grooming may have resulted in unintentional oral exposure of the antibiotic, as well as possible absorption through the skin wound. Neomycin has been demonstrated to induce antibiotic-associated diarrhea in hamsters, and the other antibiotics are not known to induce diarrhea or colitis. Unlike the other antibiotics, neomycin can be absorbed through abraded skin or wounds. The Vetropolycin-� applied repeatedly to the dorsal cervical region of the hamsters in this case contained the same mix of antibiotics: polymixin B, neomycin and bacitracin.Â
This case cautions against the topical clinical application of antibiotics to hamsters. Antibiotic therapy in hamsters is challenging, and trimethoprim sulfonamides, fluoroquinolones and chloramphenicol are generally safer antibiotic choices.Â
JPC Diagnosis:
Conference Comment:
Both TcdA and TcdB are composed of two subunits: A and B; subunit A is an N-terminal glucosyltransferase domain (GTD), and subunit B is responsible for the delivery of subunit A into the target cell. Repetitive oligopeptides on subunit B bind to sugar moieties on host cell surfaces; the toxins then enter the cell through clathrin-mediated endocytosis, and endosomal acidification induces structural changes that create a pore in the host membrane. Next subunit B is cleaved via proteolysis to release GTD into the cytosol, where it inactivates host cell GTPase by glucosylation, leading to the previously-described deleterious effects on the cell.(6)
References:
2. Best EL, Fawley WN, Parnell P, et al. The potential for airborne dispersal of Clostridium difficile from symptomatic patients. Clin Infect Dis. 2010;50:1450-1457.
3. Den+�-�ve C, Delom+�-�nie C, Barc M-C, et al. Antibiotics involved in Clostridium difficile-associated disease increase colonization factor gene expression. J Med Microbiol. 2008;57:732-738.
4. Maxie MG, Robinson WF. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 2. Philadelphia, PA: Saunders Elsevier; 2007:214.Â
5. Percy DH, Barthold SW. Hamster. In: Pathology of Laboratory Rodents and Rabbits. 3rd ed. Ames, IA: Blackwell Publishing; 2007:186-187.
6. Pruitt RN, Lacy DB. Toward a structural understanding of Clostridium difficile toxins A and B. Front Cell Infect Microbiol. 2012:2:28.