Wednesday Slide Conference, 2025-2026, Conference 6, Case 2
Signalment:
18 months old ewe, Merino, Ovis ariesHistory:
This ewe developed clinical signs including decreased menace response, visual impairment, motor deficit and reduced herding instinct. The animal was euthanized.
Gross Pathology:
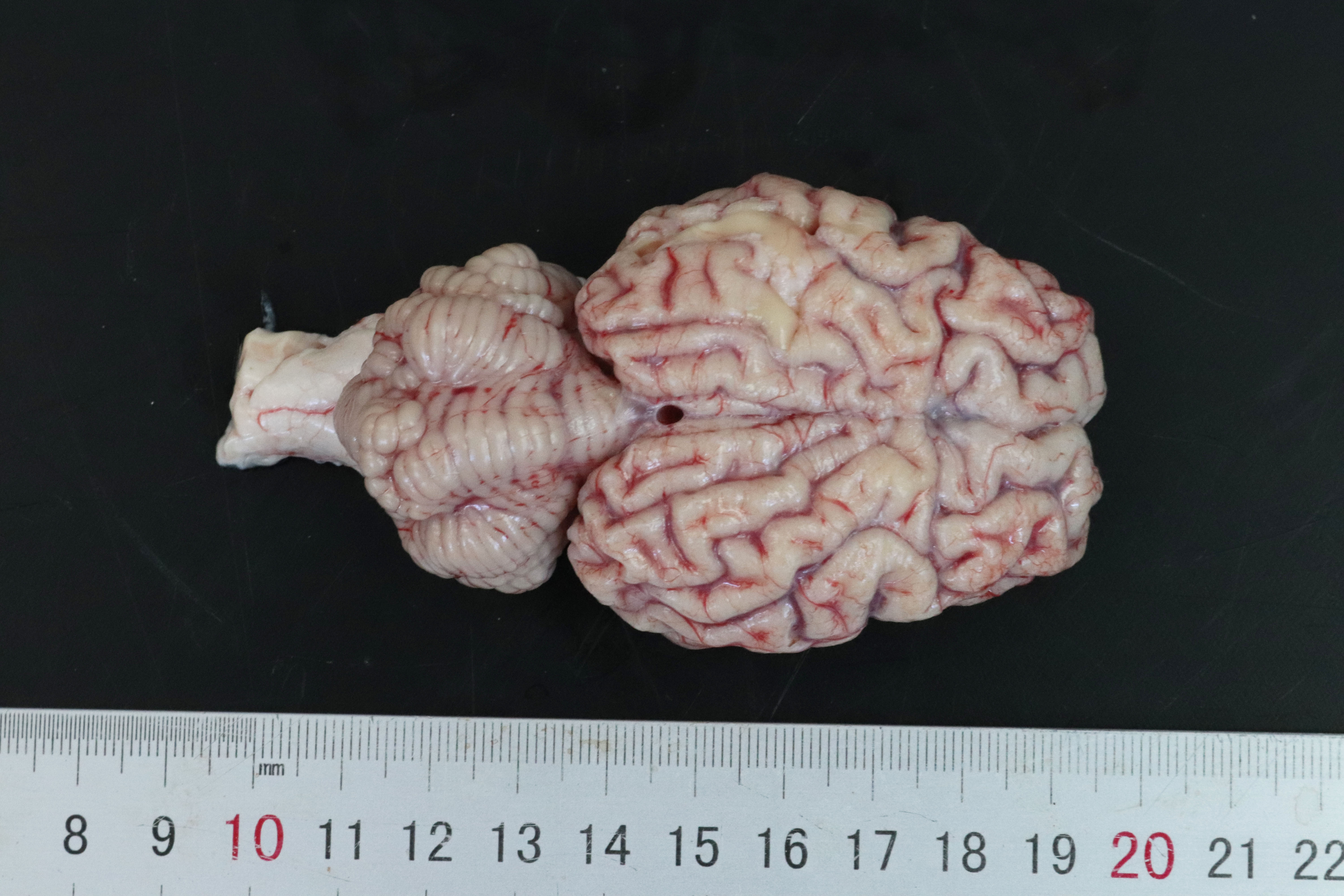
At postmortem examination the cerebral hemispheres were small, firm and flattened dorsoventrally. The sulci were wider, gyri were narrower than normal. There was diffuse, mild to moderate yellow discoloration.Laboratory Results:
Missense mutation (c.184C > T; p.Arg62Cys) in ovine CLN6 gene detected by single nucleotide polymorphism (SNP) genotyping using a commercial SNP-Chip panel.Microscopic Description:
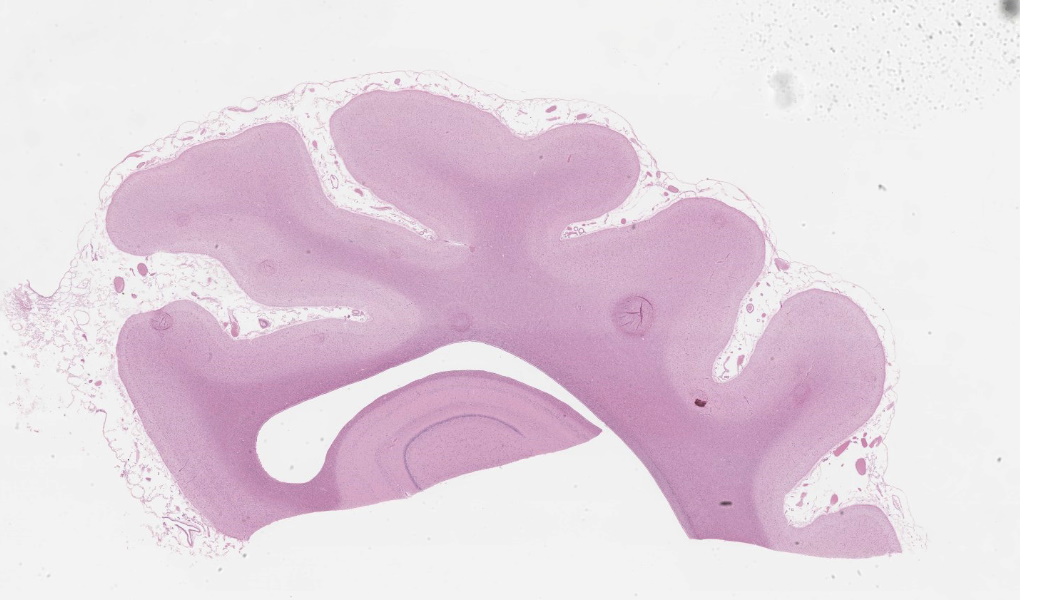
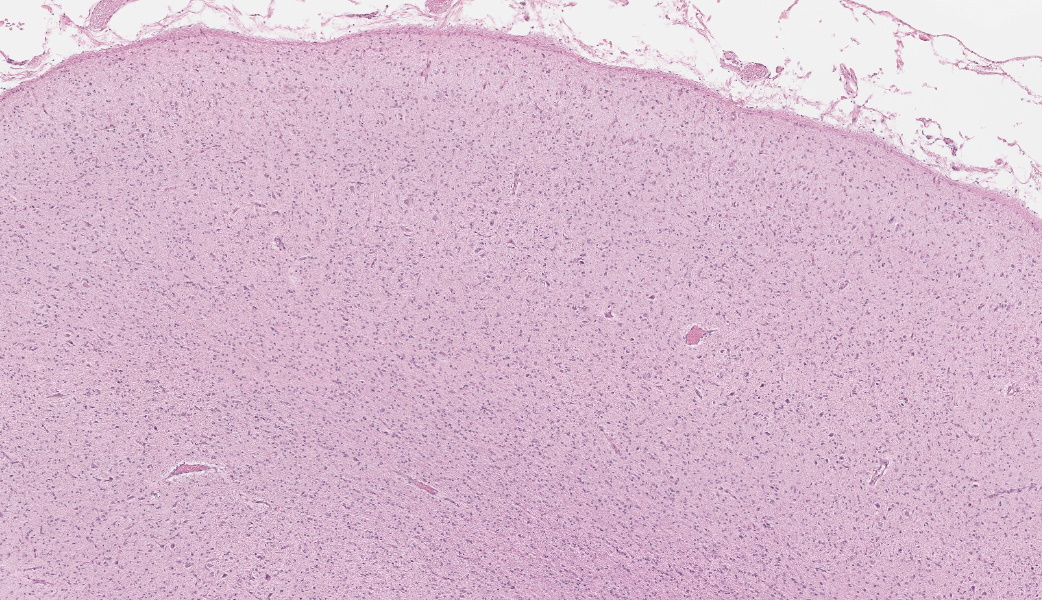
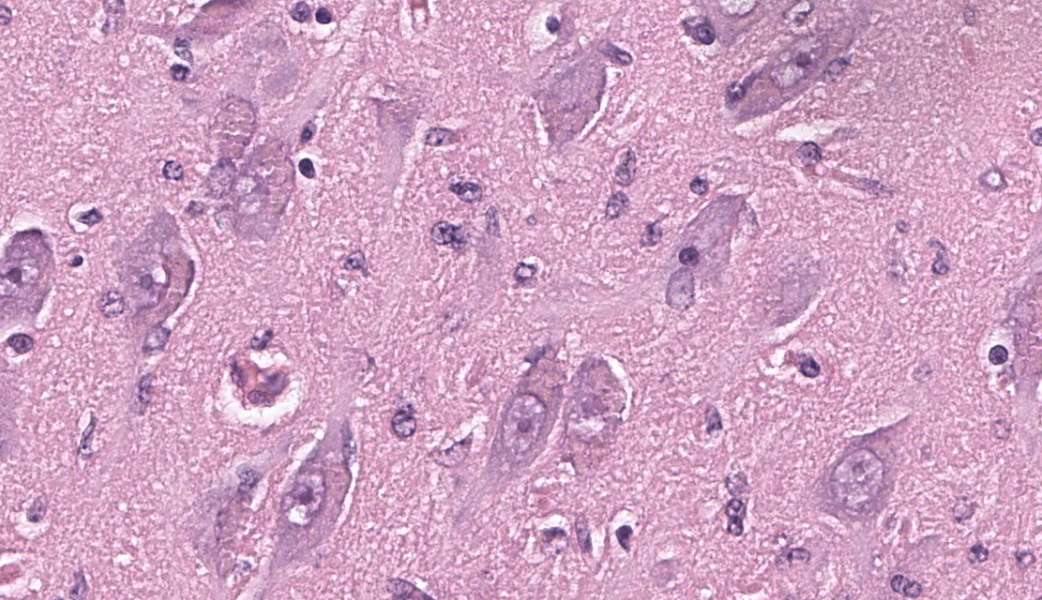
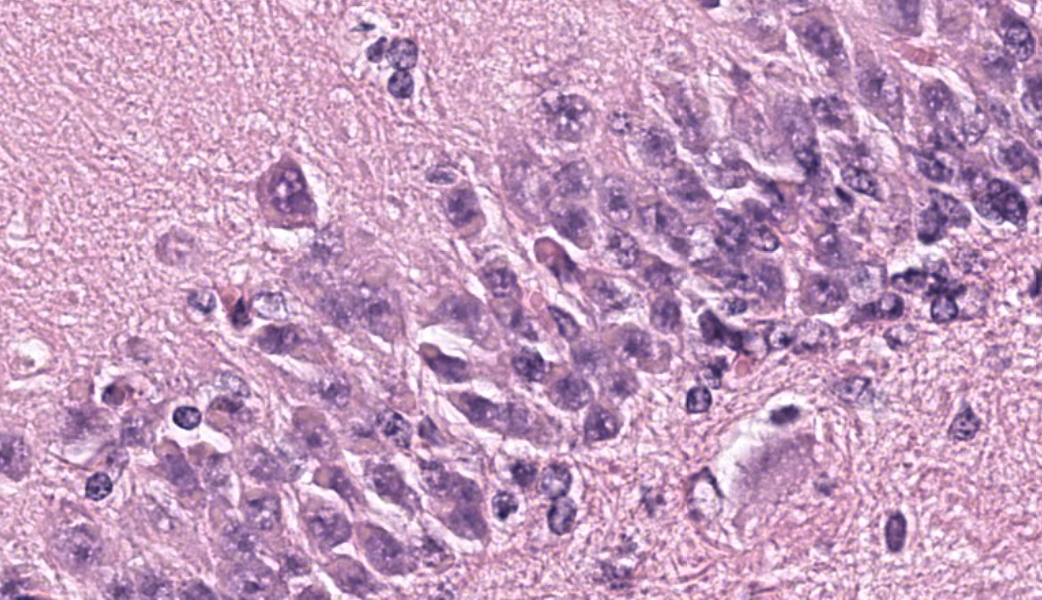
Brain, frontal cortex: There is diffuse disruption of the laminar cortical architecture and diffuse atrophy of all layers, most apparent in the granular, pyramidal and ganglionic cell layers (mid-layers atrophy). The grey matter shows neuronal loss and diffuse moderate gliosis with increased numbers of astrocytes. The remaining neurons frequently contain variable numbers of up to 5 µm diameter, granular to globular, glassy, eosinophilic, intraneuronal bodies displacing large and often vesicular nuclei. Small neurons show hypereosinophilic cytoplasmic and pyknotic nuclei (necrosis).Luxol fast blue (LFB): Intraneuronal storage material is LFB positive.
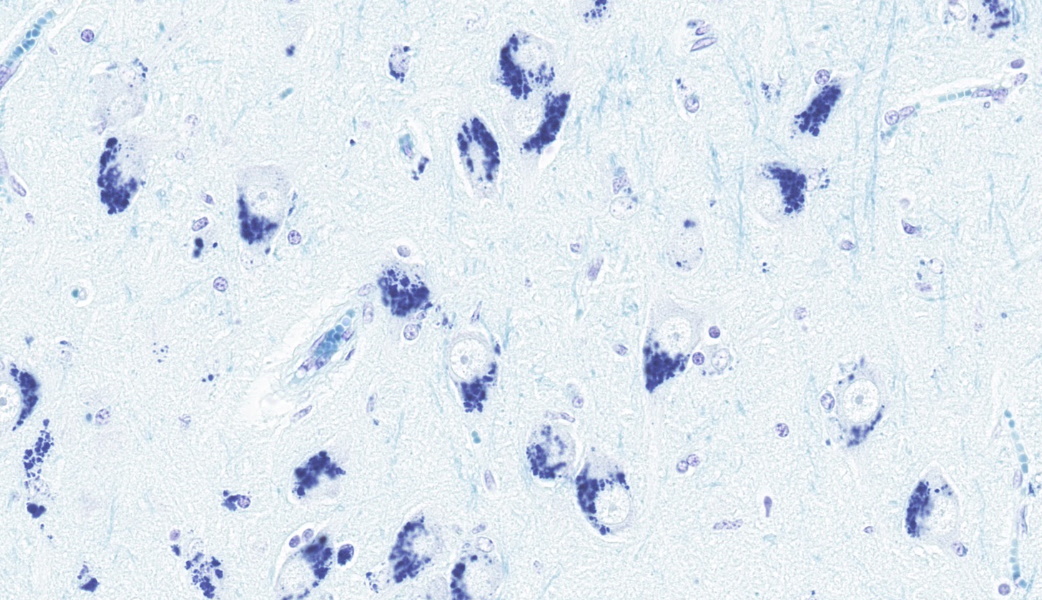
Periodic acid-Schiff (PAS): Intraneuronal storage material is PAS positive.
Contributor's Morphologic Diagnoses:
Brain, frontal cortex: Neuronal degeneration and loss, diffuse, moderate with intracytoplasmic ceroid accumulation and moderate astrocytosis, Merino, ovine.Neuronal ceroid lipofuscinosis
Contributor's Comment:
Neuronal ceroid-lipofuscinoses are a heterogenous group of recessively inherited lysosomal storage diseases. In human medicine this neurodegenerative disease is referred to as Batten's disease and affects children.1 This disease is characterized by atrophy of cerebral cortex, retina and cerebellar Purkinje system and accumulation of autofluorescent pigment in neurons and other cells. The pathogenesis of at least some forms of NCL may involve a defect in mitochondria rather than a defect in lysosomal catabolism and may involve accumulation of hydrophobic protein.2Studies show that functional neuron loss is related to selective loss of specific neuron populations that is preceded by localized glial activation, and synaptic alterations. Rather than storage material accumulation, localized activation of glia is a better predictor of the distribution of subsequent neuron loss.5,7 It is not clear whether this glial activation contributes to neuron loss or is a protective response, nevertheless it appears that neuroinflammation is a key part of the pathogenesis in many, if not all, forms of NCL.10
NCLs have been documented in a number of domestic animal species, including dogs, cats, cattle, and several breeds of sheep such as Merino, South Hampshire, Rambouillet and White Swedish Landrace.3,6,9,11 Affected Merino sheep developed clinical signs between 7 months and a year of age. The ovine gene organization in this breed is very similar to that of human CLN6 (late infantile variant) and there is a high degree of homology between species along the whole coding length.4 Active research studies utilize sheep flocks containing affected animals deliberately bred and maintained for studies relevant to human disease.
Contributing Institution:
The University of Sydney, University Veterinary Teaching Hospital Camdenhttps://www.univetscamden.com.au/
Veterinary Pathology Diagnostic Services
http://sydney.edu.au/vetscience/vpds/
JPC Diagnoses:
Cerebrum: Neuronal degeneration and loss, chronic, diffuse, severe, with cortical atrophy, neuronal intracytoplasmic pigment, mild astrocytosis, and hydrocephalus ex vacuo.JPC Comment:
This classic case of ovine neuronal ceroid lipofuscinosis (NCL) stimulated great discussion on some of the histologic changes seen in the conference slide. It was the opinion of conference participants that there was true ventricular dilation evident histologically, likely secondary to the cerebral atrophy and parenchymal loss that is characteristic in this condition, leading to the development of a hydrocephalus ex vacuo. This brand of hydrocephalus is characterized by the ventricles filling with additional cerebrospinal fluid (CSF) in response to a vacuum created by the extra space from parenchymal loss. As the pathogenesis would imply, there is no accompanying increase in CSF pressure with this type of hydrocephalus as there is in other types.One participant remarked that there was an increased number of visible axons within the affected grey matter. This posed the question of whether the increased visibility of axons was secondary to the neuronal loss from NCL or if there was true disorganization of the axons from the outset. The jury was split on this one, but the majority thought it was likely due to the former. A discussion on the intracytoplasmic storage material within neurons in this condition followed, including a review of the difference between lipofuscin and ceroid. Lipofuscin is considered a normal “wear and tear” pigment seen in long-lived cells and is not considered to be related to pathologic processes. Ceroid, however, is only seen in disease states and accumulates secondary to oxidative stress. The autofluorescent proteins in these cases are stored within lysosomes, which are unable to further break them down due to an endo-lysosome defect. In this case, in-house Luxol fast blue and PAS stains were performed, which nicely highlighted the intracytoplasmic accumulations.
Currently, there are 13 different forms of NCLs documented in humans.9 Each one has a distinct single-gene defect that affects the encoding of proteins in the endolysosomal system. These forms are generally classified into either soluble lysosomal enzyme/cytosolic protein deficiencies or insoluble transmembrane protein defects.9 The lysosome is traditionally viewed as the ‘waste-disposal’ unit of the cell, but recent studies on NCLs has shown that the lysosomal system also plays critical roles in nutrient sensing and cellular homeostasis. This indicates that NCL mutations have wide effects on cellular functions in multiple organ systems, including autophagy and synaptic dysfunctions.9 The use of animal models for NCLs has enabled researchers to identify the most susceptible neuronal populations and has demonstrated that glial cells are both negatively affected by and actively contribute to disease progression.9
References:
- Anderson G, Elleder M, Goebel HH. Morphological diagnostic and pathological considerations. In: In: Mole SE, Williams RE, Goebel HH, ed. The Neuronal Ceroid Lipofuscinoses (Batten Disease). 2nd ed. New York, NY: Oxford University Press; 2011:35-36.
- Cantile C, Youssef, S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 1. 6th ed. Louis, MO: Saunders Elsevier; 2016:290-291
- Chalkley MD, et al. Characterization of neuronal ceroid-lipofuscinosis in 3 cats. Vet Pathol. 2014;51(4):796-804.
- Chalkley MD, et al. Characterization of neuronal ceroid-lipofuscinosis in 3 cats. Vet Pathol. 2014;51(4):796-804.
- Cook RW, Jolly RD, Palmer DN, Tammen I, Broom MF, McKinnon R. Neuronal ceroid lipofuscinosis in Merino sheep. Aust Vet J. 2002;80:292–297.
- Cooper JD, Russell C, Mitchison HM. Progress towards understanding disease mechanisms in small vertebrate models of neuronal ceroid lipofuscinosis. Biochim Biophys Acta. 2006;1762(10):873-889.
- Jolly RD, Martinus RD, Palmer DN. Sheep and other animals with ceroid-lipofuscinoses: their relevance to Batten disease. Am J Med Genet. 1992;42(4):609–614.
- Kay GW, Palmer DN, Rezaie P, Cooper JD. Activation of non-neuronal cells within the prenatal developing brain of sheep with neuronal ceroid lipofuscinosis. Brain Pathol. 2006;16(2):110-116.
- Nelvagal HR, Lange J, Takahashi K, Tarczyluk-Wells MA, Cooper JD. Pathomechanisms in the neuronal ceroid lipofuscinoses. Biochim Biophys Acta Mol Basis Dis. 2020;1866(9):165570.
- O’Brien DP, Katz ML. Neuronal ceroid lipofuscinosis in 3 Australian Shepherd littermates. J Vet Intern Med. 2008;22(2):472–475.
- Palmer DN, Barry LA, Tyynelä J, Cooper JD. NCL disease mechanisms. Biochim Biophys Acta. 2013;1832(11):1882-93.
- Palmer DN, Tammen I, Drogemuller C, et al. Large animal models. In: Mole SE, Williams RE, Goebel HH, ed. The Neuronal Ceroid Lipofuscinoses (Batten Disease). 2nd ed. New York, NY: Oxford University Press;2011:284–320.