Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 7
9 October 2019
Dr. John Cullen, VMD, PhD, DACVP, FIAT
Professor, Anatomic Pathology
College of Veterinary Medicine
North Carolina State University
Raleigh, North Carolina
CASE I: AP19-00238-9 (JPC 4135938).
Signalment: 12-year-old female spayed domestic longhaired cat (Felis catus)
History: The patient presented to the NCSU Small Animal Emergency Service on 1/19/19 with a
history of steady decline over the past several months with weight loss, anorexia, and vomiting. Over the last few days, the patient had also developed neurologic signs. The primary veterinarian's bloodwork found elevated liver enzymes (ALT of 166, AST of 124, ALP of 311, total bilirubin of 2.8, and conjugated bilirubin of 1.5), a mildly elevated SDMA (16, reference 0-14 ug/dl), mildly reduced BUN (15, reference 16-37), and a mildly reduced creatinine (0.8, reference 0.9-2.5). Abdominal ultrasound identified bilateral chronic nephropathy with small nephroliths or dystrophic mineralization and a splenosystemic collateral vessel. The primary veterinarian treated with a renal diet, ursodiol, and lactulose. The owners felt clinical signs did not improve. On physical exam at NCSU, she had a reduced BCS and moderate muscle wasting and was quiet and depressed with intermittent responsiveness. Due to concern for the quality of life, euthanasia was elected.
Gross Pathology: Arising from the splenic vein and connecting to the caudal vena cava in the region of the left kidney is a large shunt vessel up to 4 mm in diameter. Additionally, connecting the portal vein and caudal vena cava in the cranial abdomen are two small (1 mm in diameter), tortuous vessels. The liver is mildly reduced in size, weighing 67 g (2.9% of total body weight; normal is 3-4). The liver is diffusely markedly pale red to tan and moderately firm. Over the diaphragmatic surface of the right lateral liver lobe is a 2.0 x 0.8 cm region of hemorrhage and mild depression. The parenchyma subjacent to this focus is markedly firm. The right kidney is mildly reduced in size compared to the left kidney and has multifocal chronic infarcts up to 2 cm in diameter. No abnormalities are identified externally in the brain.
Laboratory results: No additional findings
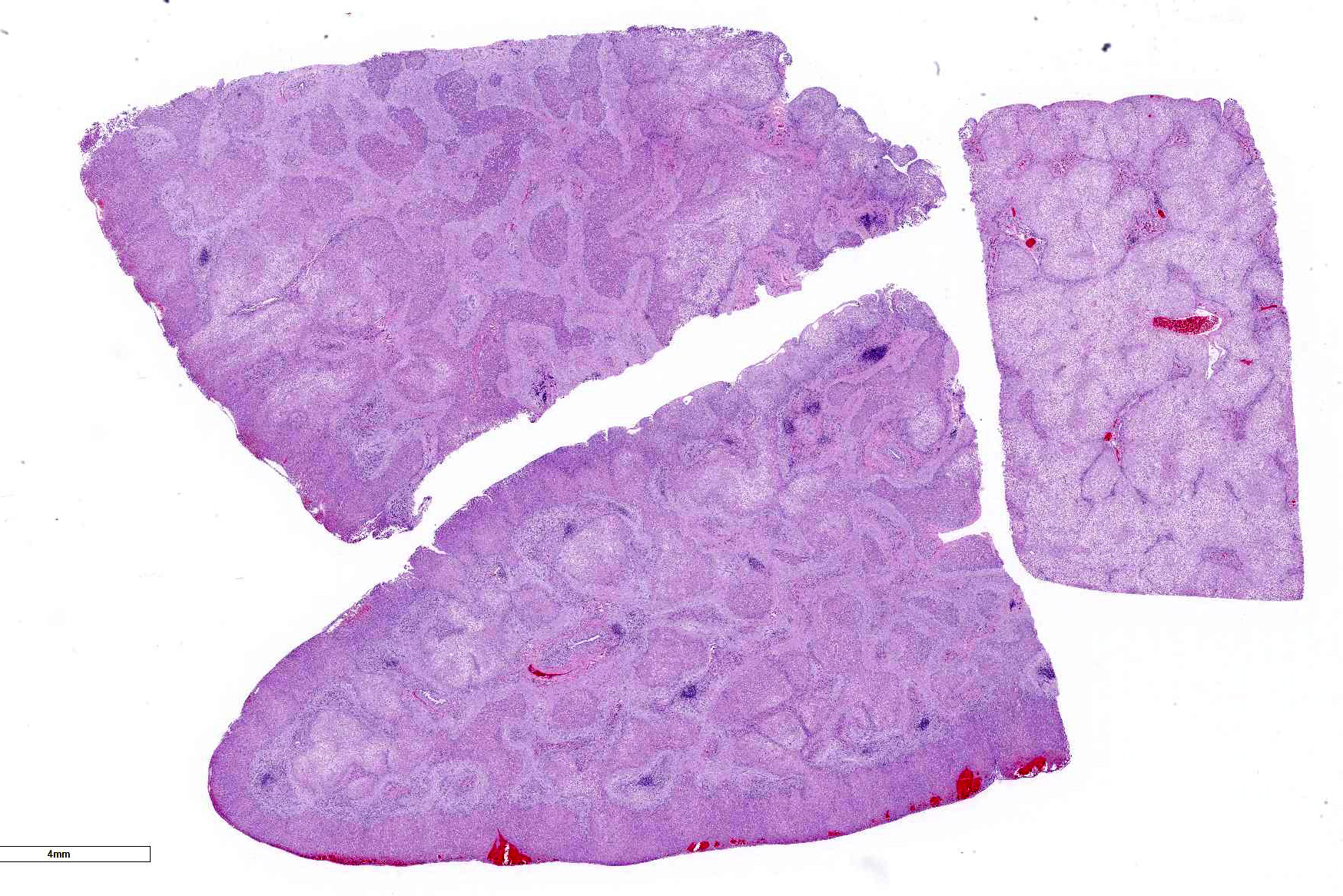
Microscopic Description:
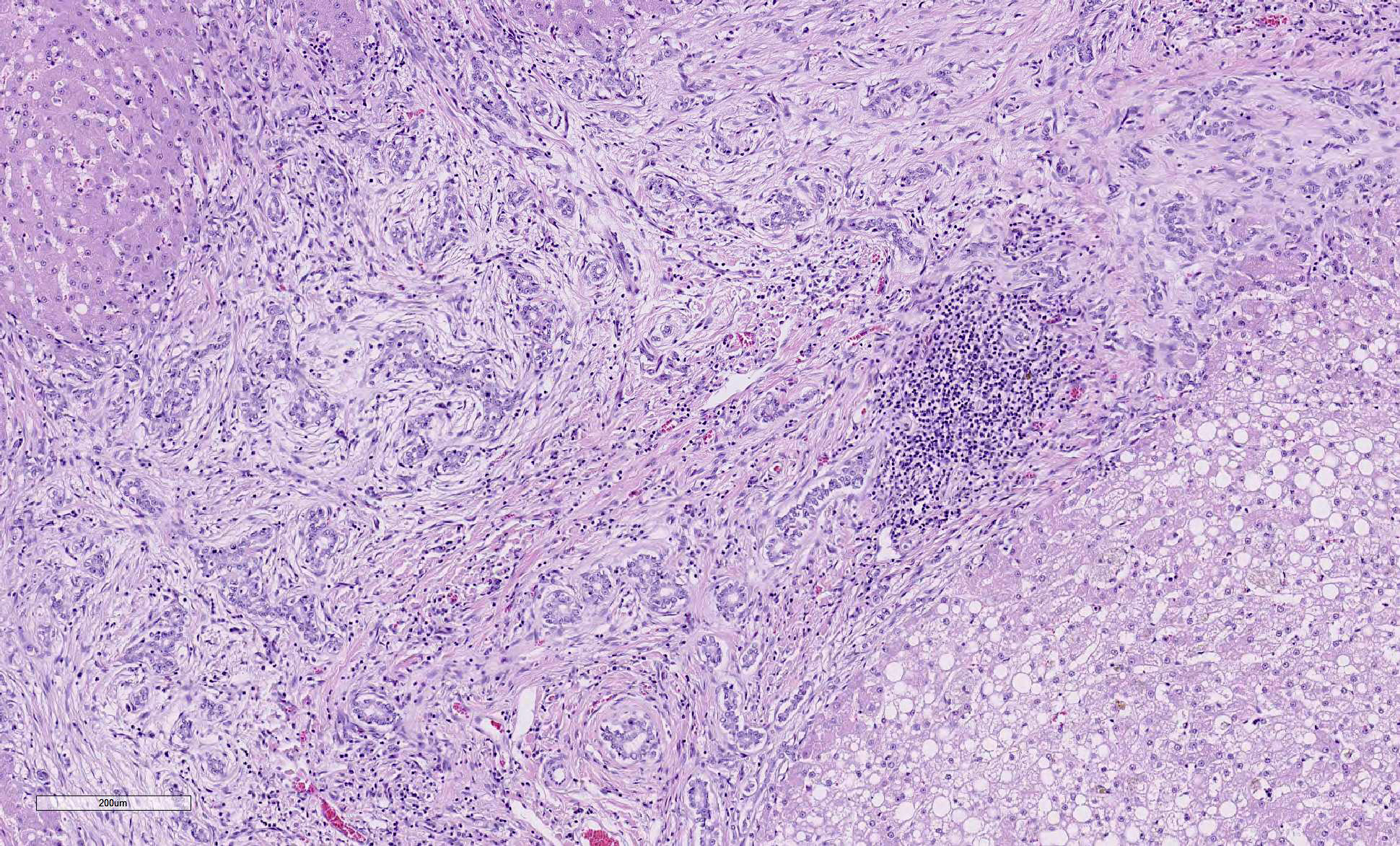
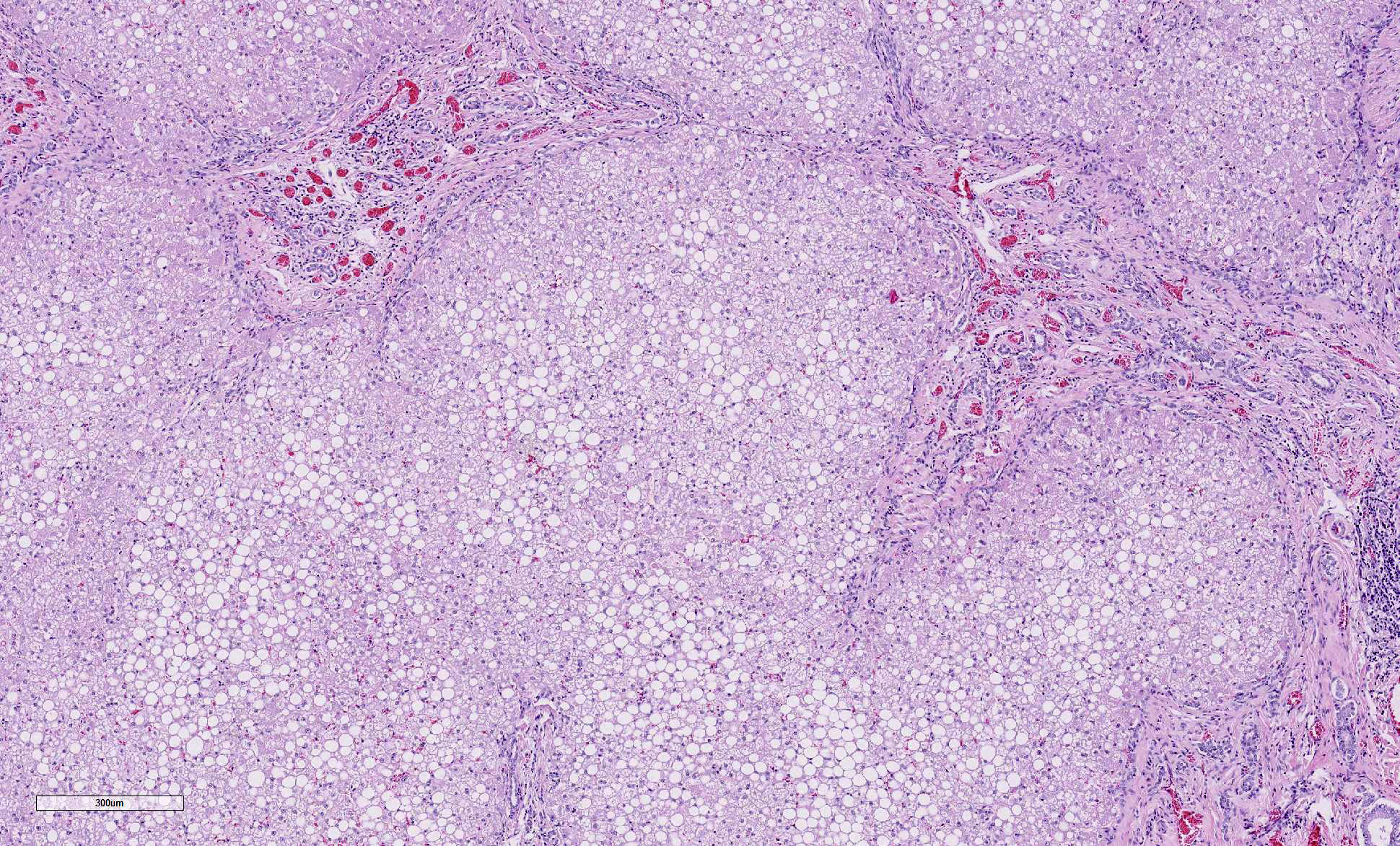
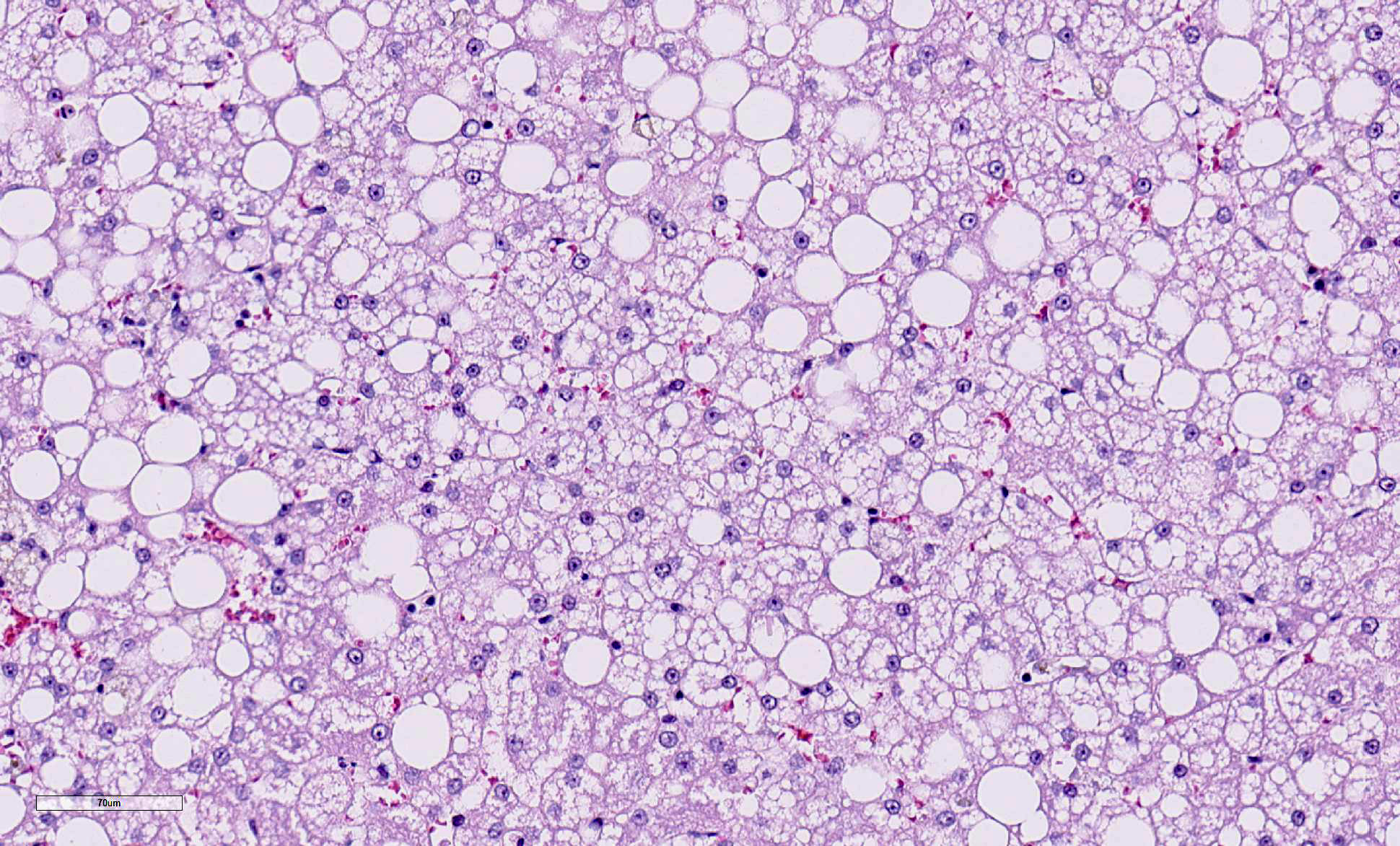
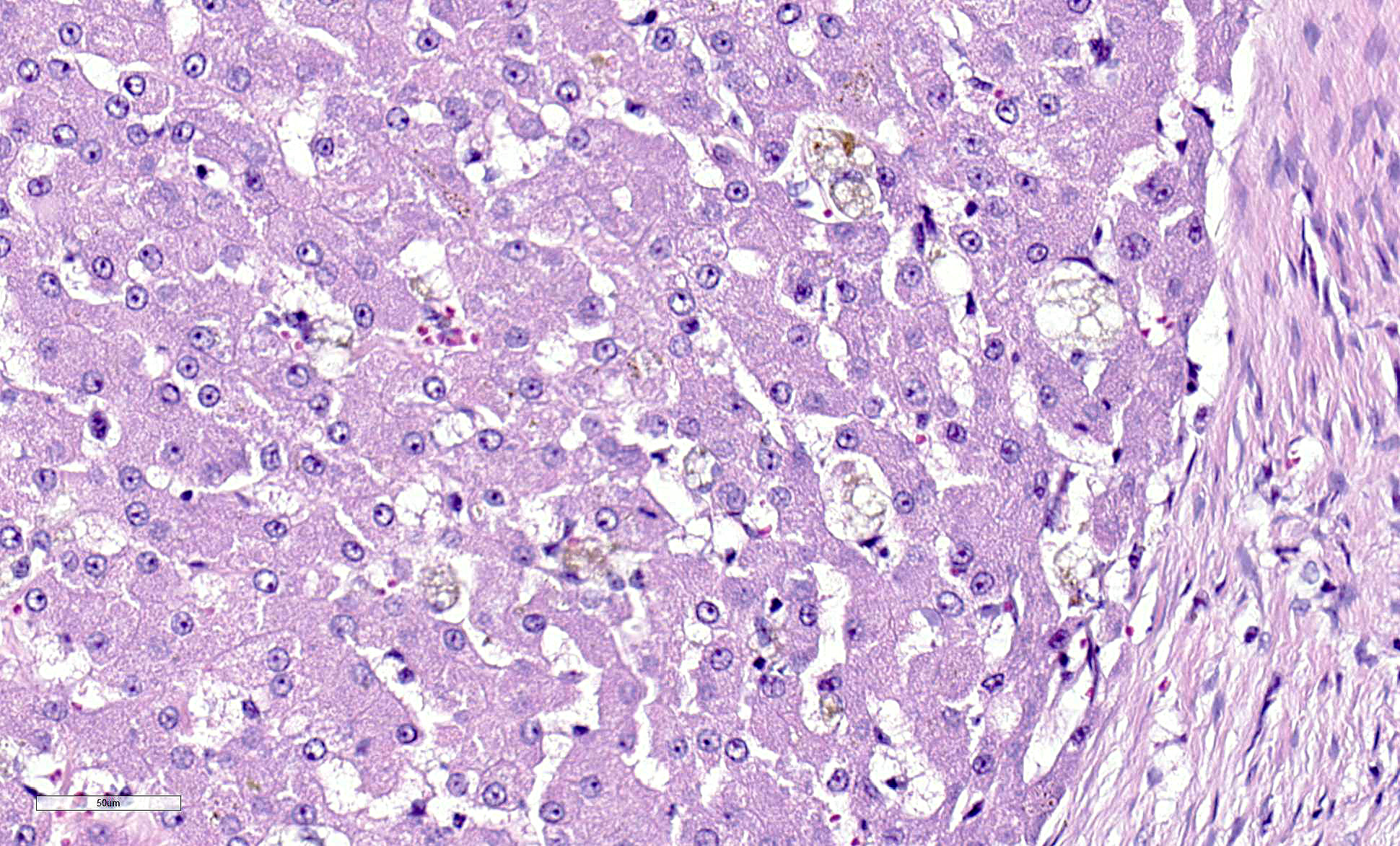
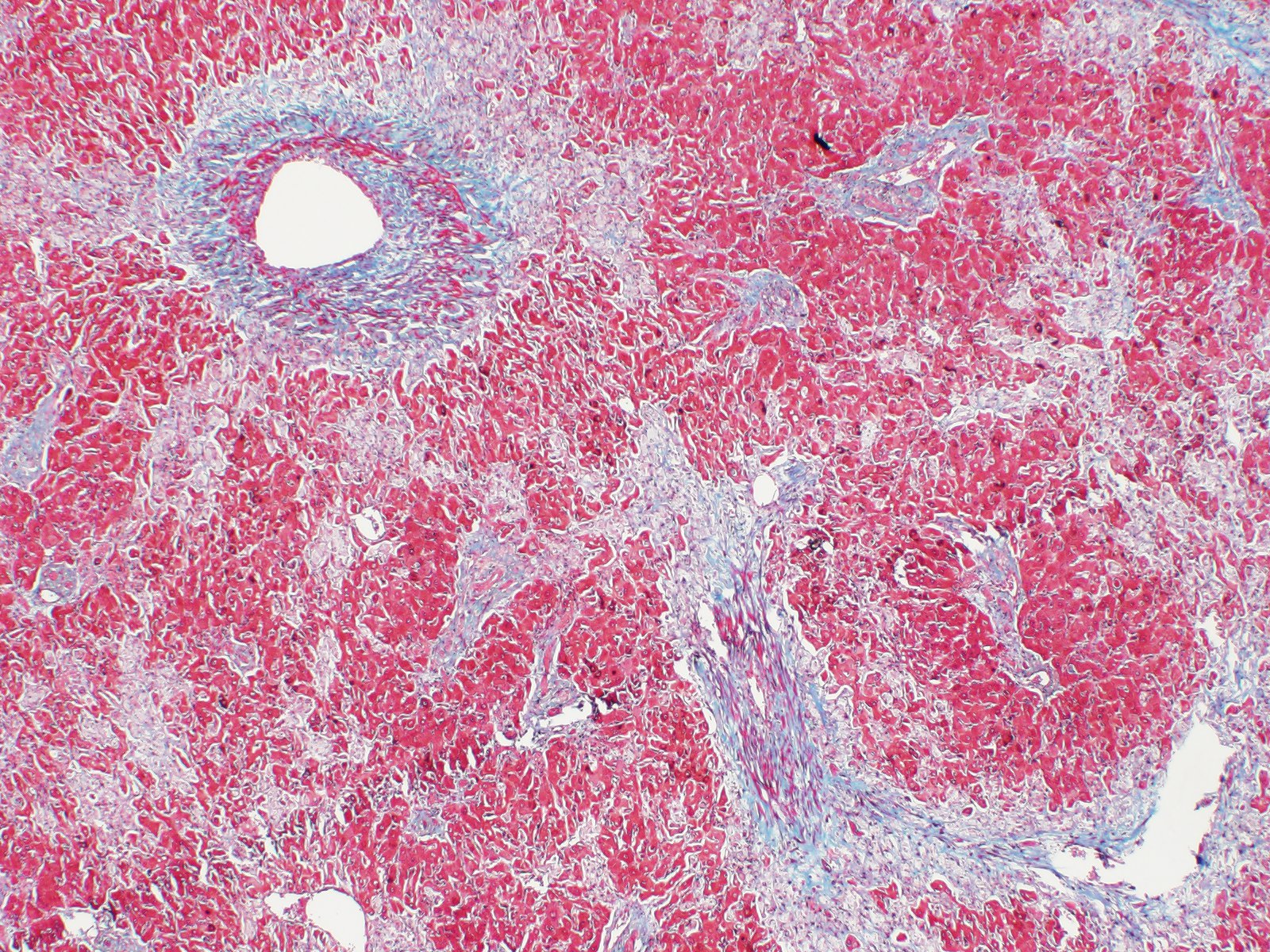
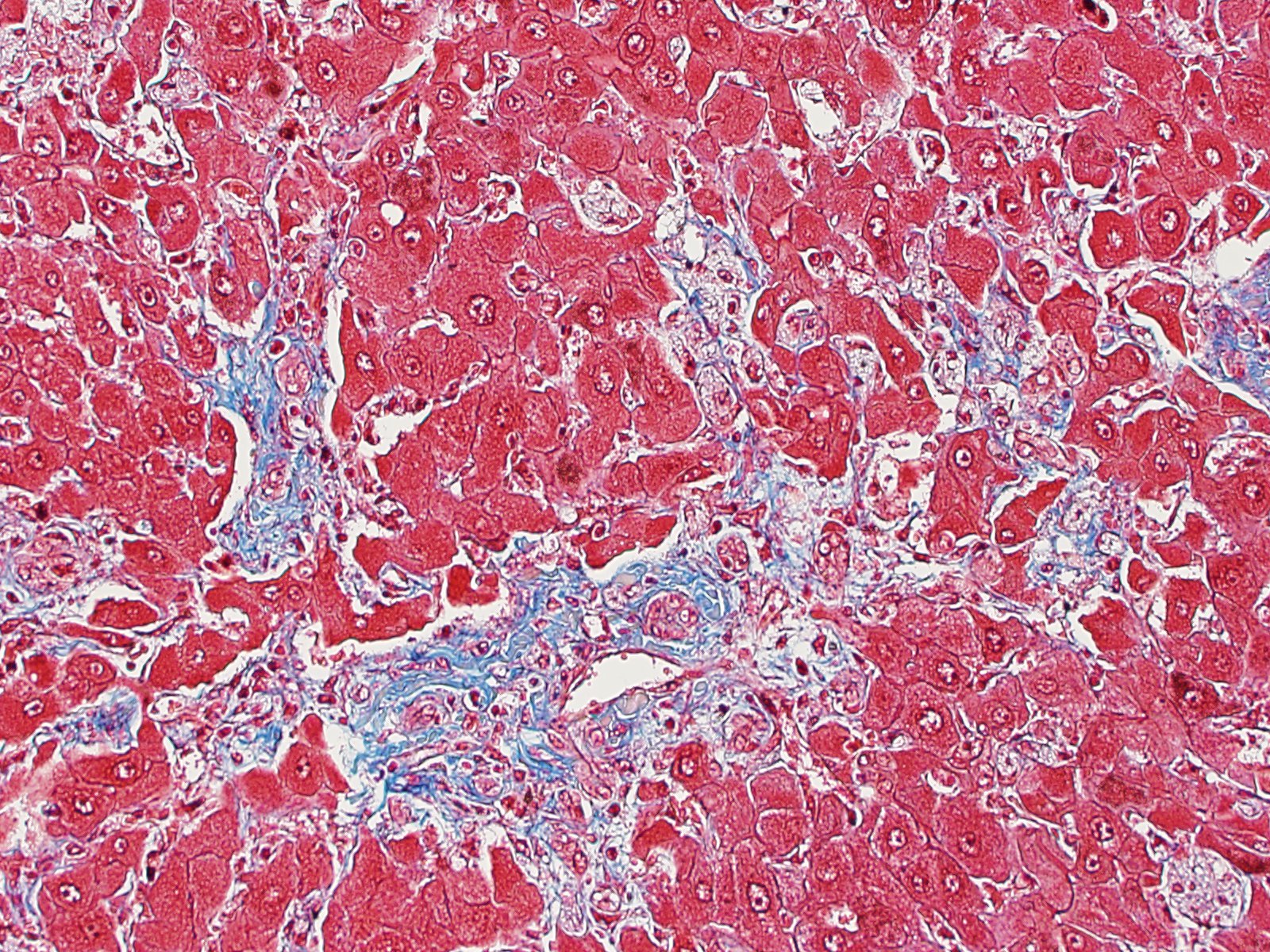
Liver: Replacing 20-50% of the hepatic parenchyma is robust, portal-to-portal bridging mature collagenous stroma, and within this stroma are markedly increased numbers of small caliber bile duct profiles. Bile ducts often have irregular to absent lumina, are tortuous with occasional branching, and frequently occur at the limiting plate directly adjacent to periportal hepatocytes. Mildly increased numbers of arteriole profiles accompany hyperplastic bile ducts. Portal veins are frequently reduced in diameter or absent. Within this bridging fibrosis are mild, multifocal infiltrates of lymphocytes and plasma cells. In the subcapsular region, sinusoids are multifocally markedly congested. The mesothelium is regionally hypertrophied. Variably 15-75% of hepatocytes, in a patchy predominantly centrilobular to midzonal distribution, contain one to multiple, small to large, discrete, clear, lipid type vacuoles. Scattered within the parenchyma are occasional pigment granulomas composed of foamy macrophages that are occasionally laden with golden-brown pigment (suspected hemosiderin).
Contributor Morphologic Diagnosis:
Liver:
a. Marked, portal-to-portal bridging fibrosis with marked ductular reaction, portal vein hypoperfusion, mild arteriolar hyperplasia, and mild lymphoplasmacytic chronic hepatitis (consistent with congenital hepatic fibrosis)
b. Moderate, multifocal to coalescing, hepatic lipidosis
Contributor Comment: The clinical history, gross findings, and histologic findings in this case, are all consistent with congenital hepatic fibrosis (CHF). CHF is a condition resulting from abnormal development at the ductal plate affecting small interlobular bile ducts.3,10 CHF is
histologically characterized by periportal to bridging fibrosis with numerous small often irregular bile ducts and a reduction in the number of portal vein branches.3 In humans, the fibrosis has been shown to be progressive.10 Inflammation and cholestasis are generally absent, and regenerative hyperplasia is not typically a feature.3 Sequelae include portal hypertension, ascites, and acquired extrahepatic portosystemic shunts. These signs may be the result of progressive fibrosis or altered and insufficient portal vein architecture.3
CHF is a morphologic diagnosis but not a single clinical entity and instead is the result of a spectrum of conditions that affect the ductal plate.4 In humans, isolated CHF is rare but reported, and CHF is more often associated with fibrocystic diseases (FCDs), including polycystic kidney disease (PKD).10 While inheritance is most commonly autosomal recessive, X-linked and autosomal dominant inheritance are also described. Notably, although FCDs are currently categorized by phenotype, in the future, gene-based classification may significantly alter distinction among the FCDs.10
In the veterinary literature, there are relatively few reports of CHF. Species in which CHF has been reported include dogs3,14,17, cats1,19, aborted and neonatal calves2,18, foals including Swiss Freiberger horses and multiple other breeds5,11,12, an African green monkey17, and a colony of Sprague-Dawley rats now used as a model for human autosomal recessive CHF15. In a case series of five dogs, all dogs presented at one year of age or younger with signs of liver disease.3 Several other series of hepatic/hepatoportal fibrosis in young dogs have been published but do not expressly diagnose CHF versus other causes of hepatic fibrosis.14,16
There are two reports describing CHF in cats. One report describes CHF in cats with polycystic kidney disease (PKD), an autosomal dominant disorder for which Persian cats are predisposed.1 Among cases of PKD, 28% had CHF, and 17% had both CHF and liver cysts. The age range for CHF-affected cats in this report was 1 to 13 years, and one of those cats had clinical signs of liver disease. 3 Additionally, there is a report of two cats with CHF and secondary acquired portosystemic shunts, and in this report, both cats also had evidence for concurrent PKO, as well as in one cat a concurrent congenital portosystemic shunt.19 Interestingly in the case presented here, the cat lacked gross and histologic evidence of PKD, suggesting that in contrast to what is reported in the literature, CHF is not exclusively a manifestation of PKD in cats. Additionally, this case demonstrates that in contrast to dogs, CHF may progress more slowly in cats than in dogs and not become clinically relevant until later in life.
Contributing Institution:
North Carolina State University College of Veterinary Medicine
https://cvm.ncsu.edu/research/departments/dphp/programs/pathology/
JPC Diagnosis: Liver: Fibrosis,
portal and bridging, diffuse, severe, with marked biliary hyperplasia,
hepatocellular loss, nodular hepatocellular regeneration, and hepatocellular
lipidosis.
JPC Comment:
In humans, congenital hepatic fibrosis (CHF) is an autosomal recessive disease resulting from a mutation on PKDH1, whose gene product encodes fibrocystin/polyductin, a ciliary protein expressed in cholangiocytes as well as renal tubular epithelium.6 In humans, CHF is part of the fibropolycystic diseases (FCDs), which also include autosomal dominant and autosomal recessive polycystic kidney disease, Caroliâs disease, and von Meyenburg complexes (biliary hamartomas).6,9 While a rare condition in humans (estimated in 1 in 20,000 live births) it often occurs with diseases of other organs as well as other congenital diseases, including Joubert Syndrome and Bardet-Biedl Syndrome.6
In most other conditions resulting in progressive hepatic fibrosis, fibrosis
is a reparative response to a previous necrotizing or inflammatory insult.6,9
In CHF, fibrosis is genetic, resulting from lack of remodeling of the ductal
plate, the embryologic precursor to the intrahepatic bile ducts. The lack of
remodeling ultimately results in the persistence of immature biliary
structures, cystic malformation, and prominent peribiliary fibrotic responses.
The actual function of polycystin is not known is thought to be involved as a
regulator of transcription of various proteins involved in proliferation,
differentiation, tubulogenesis and cell-matrix interactions.6,9
The clinical spectrum of CHF in humans is extremely broad and depends largely
on whether polycystic disease is present in other organs. Cases of âpureâ CHF
may remain undetected into middle age. One of the most important consequences
of fibropolycystic disease in humans is the development of portal hypertension,
which often results in clinical symptoms of hematemesis and melena in 30-70% of
cases; clinical signs related to cholangitis are less common. which may
ultimate require hepatic resection or transplantation. Portal hypertension in
such cases ultimately may require hepatic resection or transplant.6,9
The contributor discusses a previous report of cats with CHF and PKD. Hepatic fibrosis has been reported in between 22-48% of cats with feline polycystic kidney disease, although clinical signs associated with liver failure are rare.8 Genetic testing for PKD became available following that report, and was found to be associated with a C>A mutation in exon 29 of PKD1 (polycystic kidney disease 1). In a feline case of combined CHF and PKD1 reported in 2015, genetic testing revealed a wild-type sequence at this position, suggesting an alternate pathogenesis, or perhaps a mutation not yet described in the cat, and is similar to the case described herein which PKD was not a concurrent condition.8
Upon close inspection of the submitted sections, the moderator commented on the
zone of exclusion of relatively unaffected tissue. This is supplied by a subset
of bile ducts of the smallest caliber at the periphery of the liver lobe,
suggesting that the process is affecting ducts of a particular size, such as
the lobular and interlobular ducts rather than the smallest ductules which
supply the sublobular case. The lack of pigmented macrophages suggests a
limited amount of continuing damage to bile ducts at this pint in the
development of this lesion. The moderator interpreted the nodularity of the
parenchyma as simple entrapment of hepatocytes without the formation of
regenerative nodules (which are not commonly seen in cats.)
The moderator commented that hepatic fibrosis is an uncommon finding in cats except for this particular condition. In dogs, the diseases generally results in death within 6-10 months, but cats may go on for years. Dogs develop considerable portal hypertension which does not appear to be of great clinical importance in affected cats (which also much less commonly result in the development of acquired portosystemic shunts than in dogs).
References:
1. Bosje JT, Van den lngh TS GAM, van der Linde-Sipman JS. Polycystic kidney
and liver disease in cats. Veterinary Quarterly. 1998;20(4), 136-139.
2. Bourque AC, Fuentealba IC, Bildfell R, Daoust PY, Hanna P. Congenital hepatic fibrosis in calves. Can Vet J. 2001 ;42:145-146.
3. Brown DL, Van Winkle T, Cecere T, Rushton S, Brachelente C, & Cullen JM.
Congenital hepatic fibrosis in 5 dogs. Vet Pathol. 2010;47(1):102-107.
4. Desmet VJ. What is congenital hepatic fibrosis?. Histopathology. 1992;20(6),465- 478.
5. Drogemuller M, Jagannathan V, Welle M, et al. Congenital hepatic fibrosis in the Franches-Montagnes horse is associated with the polycystic kidney and hepatic disease 1 (PKHD1) gene. PLoS One. 2014;9:e110125.
6. Fabris L, Fiorotto R, Spirli C, Cadamuro M, Mariotti V, Perugorria MJ, Bnales JM, Srazzabosco M. Pathobiology of inherited biliary diseases: a roadmap to understand acquired liver diseases. Nat Rev Gastroenterol Hepatol 2019; 16(8):497-511.
7. Giouleme 0, Nikolaidis N, Tziomalos K, et al. Ductal plate malformation and
congenital hepatic fibrosis: clinical and histological findings in four
patients. Hepatology Research. 2006;35(2):147-150.
8. Guerra JM, Daniel AGT, Cardoso NC, Grandi F, Quieroga F, Cogliati B. Congenital hepatic fibrosis and polycystic kidney disease not linked to C>A mutation in exon 29 of PKD1 in a Persian cat. J Fel Med Surg Open Rep 2015; doi: 10,1177, 2055116915619191
9. Guerra JA, Kampa KC, Zapparoli M, Alves VAF, Ivantes CAP. Congenital hepatic fibrosis and obliterative portal venopathy without portal hypertension â a review of literature based on an asymptomatic case. Arch Gastroenterol 2018; doi: 10.1590/S0004-2803.201800000-91.
10. Gunay-Aygun M, Gahl WA, Heller T. Congenital hepatic fibrosis overview.
In: GeneReviews®[lnternet]. University of Washington, Seattle, 2014. Accessed 14 Apr 2019 at< https://www.ncbi.nlm.nih.gov/books/NBK2701/>.
11. Haechler S, Van den lngh TS, Rogivue C, Ehrensperger F, Welle M. Congenital hepatic fibrosis and cystic bile duct formation in Swiss Freiberger horses. Vet
Pathol. 2000;37:669-671.
12. Molin J, Asfn J, Vitoria A, et al. Congenital hepatic fibrosis in a purebred Spanish horse foal: pathology and genetic studies on PKHD1 gene mutations. Vet Pathol. 2018;55:457-461.
13. Rothuizen J, Van Den lngh TS, Voorhoutm G, Van Der Luer RJT, Wouda W.
Congenital porto-systemic shunts in sixteen dogs and three cats. Journal of
Small Animal Practice. 1982;23(2):67-81.
14. Rutgers HC, Haywood S, Kelly DF. Idiopathic hepatic fibrosis in 15 dogs. The
Veterinary record. 1993;133(5),115-118.
15. Sanzen T, Harada K, Yasoshima M, Kawamura Y, Ishibashi M, Nakanuma Y.
Polycystic kidney rat is a novel animal model of Caroli's disease associated with congenital hepatic fibrosis. The American journal of pathology.
2001; 158(5): 1605-1612.
16. Van TOI, Rothuizen J. Hepatoportal fibrosis in three young dogs. The Veterinary record. 1982; 110(25),575-577.
17. Wallace S, Blanchard T, Rabin L, Dick E, Allan J, Hubbard G. Ductal plate malformation in a nonhuman primate. Vet Pathol. 2009;46:84-87.
18. Yoshikawa H, Fukuda T, Oyamada T, Yoshikawa T. Congenital hepatic fibrosis in a newborn calf. Vet Pathol. 2002;39:143-145.
17. Zandvliet MM, Szatmari V, van den lngh T, Rothuizen J. Acquired portosystemic shunting in 2 cats secondary to congenital hepatic fibrosis. Journal of veterinary internal medicine. 2005;19(5),765-767.