WSC 2022-2023
Conference 25
Case II:
Signalment:
12-year-old neutered male European Shorthair cat (Felis catus)
History:
The cat had a clinical history of respiratory dyspnea and cough.
Gross Pathology:
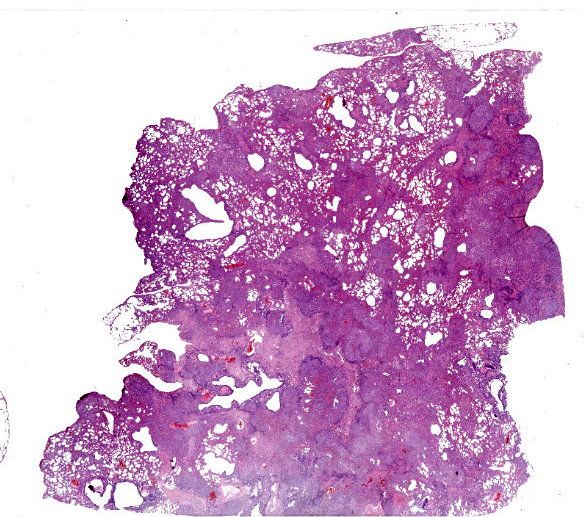
The entire pulmonary lobe was submitted for histopathology.
Laboratory Results:
No laboratory findings reported.
Microscopic Description:
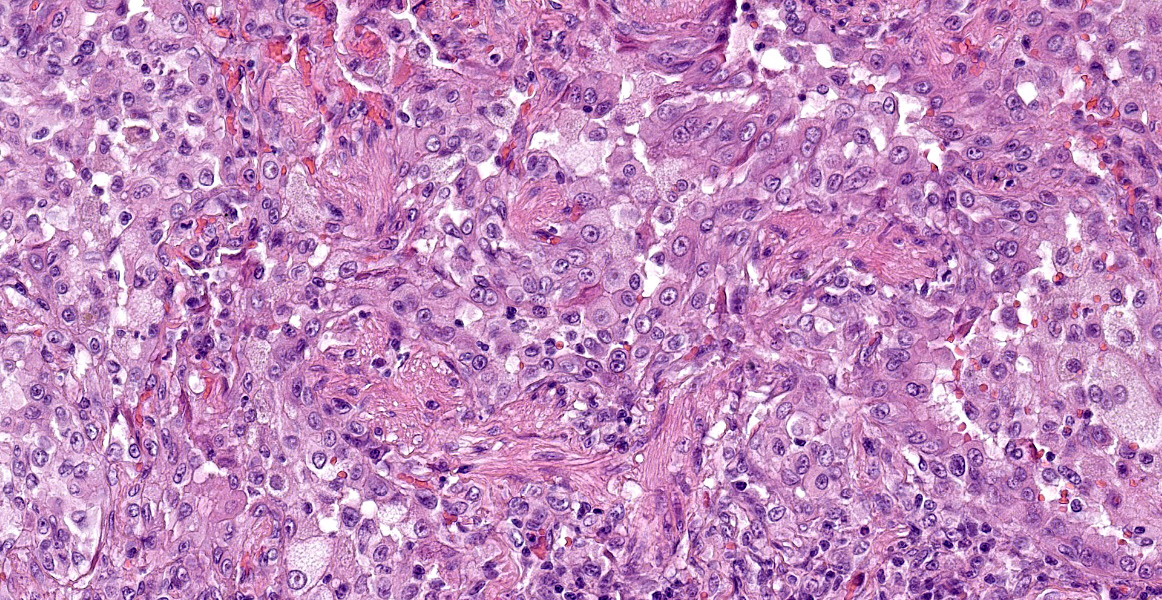
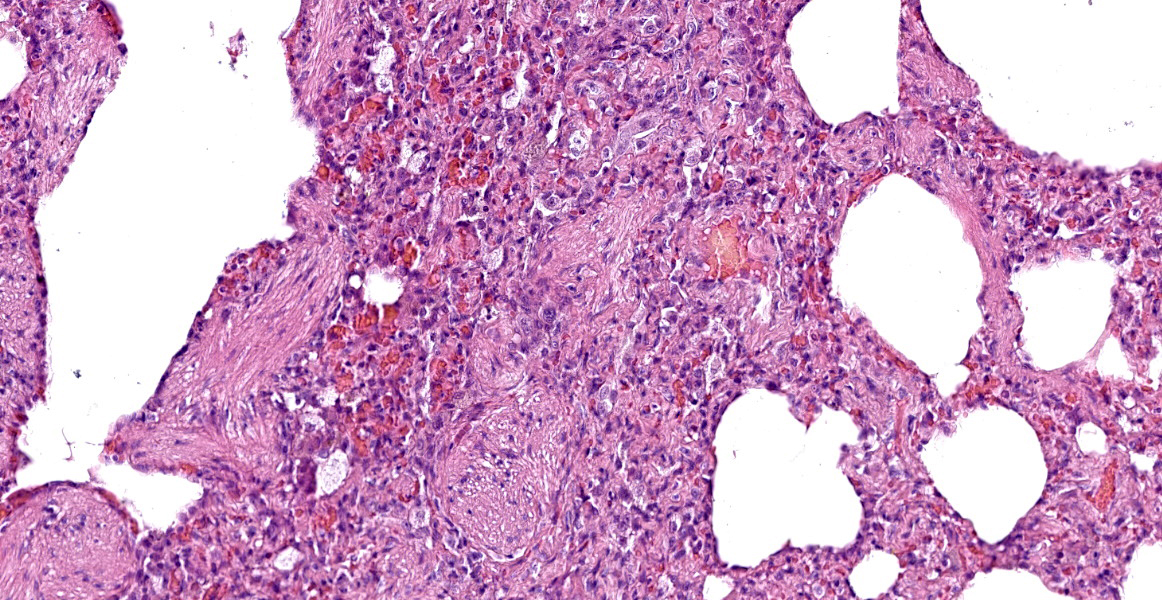
Lung. About 70% of the parenchyma is effaced by a multifocal to coalescing, severe proliferative process. Alveolar spaces are filled with numerous round cells of 25 micrometer in diameter, with distinct cell borders, high nuclear-to-cytoplasmic ratio, and small to moderate amounts of finely granular eosinophilic cytoplasm. Nuclei are round, central, with finely stippled chromatin and one or more visible nucleoli. Anisocytosis and anisokaryosis are mild and occasional mitoses with atypical features are present. Admixed to these cells, there are multifocal aggregates of large alveolar macrophages with foamy cytoplasm, or scant cell debris and hypersegmented neutrophils. In about the 20% of the section and mainly the periphery of the lesions, the alveolar spaces are multifocally dilated and merged, with disrupted and blunted septae (emphysema). Lining the alveoli there are plump and cuboidal alveolocytes (type II alveolocytes hyperplasia). Smooth muscle fibers of terminal bronchioles are diffusely increased in size (hypertrophy). The interstitium is multifocally and markedly expanded by haphazardly arranged eosinophilic collagen fibers embedding fibrocytes (fibrosis), small caliber capillaries and rare macrophages containing coarsely granular brown-black pigment (anthracosis), and numerous lymphocytes; bronchial glands are increased in number (hyperplasia) and bronchial walls are collapsed multifocally. Blood vessels are multifocally engorged with erythrocytes (hyperemia) and the tunica media of medium and small arteries is moderately thickened by several layers of smooth muscle cells (arteriolar hypertrophy).
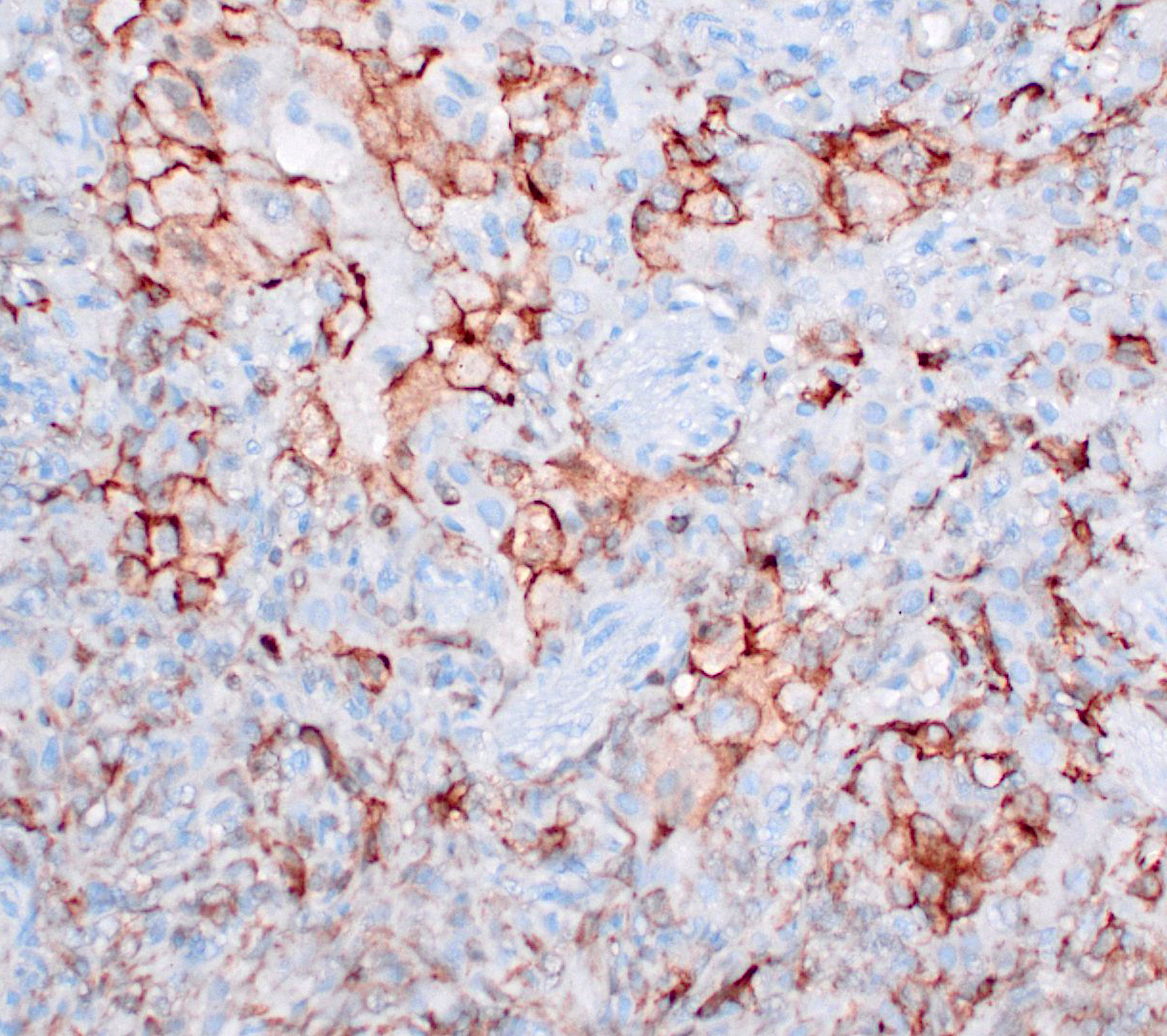
Immunohistochemistry (slides not submitted, pictures submitted as supporting material on DVD): vimentin+, Iba+, e-cadherin+, CK-.
Contributor’s Morphologic Diagnosis:
Lung. Multifocal to coalescing, alveolar histiocytosis with emphysema and smooth muscle hyperplasia, with fibrosis and arteriolar hypertrophy.
ND. Feline Pulmonary Langerhans cell Histiocytosis
Contributor’s Comment:
Feline pulmonary Langherans cell histiocytosis (FPLCH) is a rare disease of older cats (>10 years) that causes progressive respiratory insufficiency that leads to death secondary to pulmonary parenchymal infiltration with Langherans cells (LCs).1,7,9,13 Clinical signs vary from acute respiratory distress, including tachypnea, labored breathing, and open mouth-breathing, to prolonged chronic pulmonary disease.7,9,13 FPLCH is thought to represent a neoplastic process because of cellular morphological characteristics and extra- pulmonary invasion, reported in pancreas and kidney2,6, and the pulmonary one is the only form of Langherans cells proliferation described in this species, with no cutaneous manifestation reported.1
Similar to the pulmonary lesions in canine LCH, histiocytic proliferations affect first the peribronchial parenchyma, causing multinodular-to- diffuse proliferations that vary from 2-5 mm in diameter and affect all lung lobes.1 As the disease progresses, nodules coalesce, and lesions extend to the pleural surface. In advanced cases, the whole lungs tend to be affected, and all lobes appear diffusely firm.13 However, there is a higher degree of cellular and nuclear pleomorphism. Aggregates of histiocytes are usually cohesive, and proliferating cells extend from the peribronchial parenchyma into the surrounding alveolar septa and alveoli, eventually effacing the pulmonary parenchyma.
Ultrastructurally, Birbeck granules, which represent the internalized cell surface receptor langerin (CD207) cross-linked by an antibody, have been reported in the cytoplasm of proliferating histiocytic cells, confirming a Langerhans cell origin. This is in contrast to canine Langerhans cells, which do not have Birbeck granules.7,13
Immunohistochemically, histiocytic cells of feline LCH are positive for CD18 and E-cadherin and negative for CD204 in formalin-fixed tissues.9 In the current case, FPLCH was diagnosed on the basis of E-cadherin positive neoplastic cell, with variable pattern and intensity of immunolabeling among pulmonary lobes (see attached figures: 1, e-cadherin, 2 Iba1).
In a recent work by Hirabayashi et al., that accurately characterized histiocytic cell types of cats by immunohistochemistry and immunofluorescence, non-neoplastic histiocytes were mostly positive for Iba-1 and HLA-DR, while the immunoreactivity of other antibodies varied among histiocytes that resided in different organs. Dermal interstitial Dendritic Cells (iDCs) and macrophages were positive for CD204 and negative for E-cadherin. Epidermal LCs were negative for CD204 and positive for E-cadherin. However, antibodies for CD1a, langerin/CD207, and S100, which are regarded as specific markers of human LCs, were not available for detecting LCs on formalin-fixed, paraffin-embedded tissue sections of the cat, as well as those of the dog.9,12 Double-labeling immunohistochemistry of the lymph nodes revealed E-cadherin-positive CD204-negative histiocytes and E-cadherin and CD204-double positive histiocytes in the sinus, which may be veiled cells and LC-like cells, respectively.6 Neoplastic histiocytic cells were immunohistochemically positive for Iba-1 and HLA-DR in all cases of Feline Progressive Histiocytosis (FPH) and Histiocytic Sarcoma (HS). Immunoreactivity for CD204, CD163, and E- cadherin varied among cases. Of the HS cases, the neoplastic cells of more than half of cases presented with the iDC/macrophage immunophenotype (CD204+/E-cadherin-), while a minor number presented with the LC immunophenotype (CD204-/E-cadherin+), and with the LC-like cell immunophenotype (CD204+/E-cadherin+). Feline HS with the LC immunophenotype may correspond to Langerhans cells histiocytosis (LCH) or LC sarcoma (LCS) in humans.1 Among FPH cases of the study, the neoplastic cells of the majority of cases presented with the iDC/macrophage immunophenotype (CD204+/E-cadherin-) and a minor part with the LC immunophenotype (CD204-/E-cadherin+). Moreover, the epitheliotropism of neoplastic cells was reported in some E-cadherin-positive cases. In a previously reported case of FPLCH, E-cadherin expression by neoplastic cells was decreased in extrapulmonary metastatic lesions, while in one of the HS cases in the Hirabayashi study, the E-cadherin immunoreactivity of neoplastic cells varied in different organs.2,6 These results suggest that the expression of E-cadherin by feline neoplastic histiocytes could be altered by their microenvironments.
PLCH in humans has been associated with tobacco smoke, especially in young adults.1,3 Tobacco smoke supposedly causes damage to the bronchial epithelium, which prompts the release of peptides that stimulate alveolar macrophages to secrete cytokines that activate resident antigen-presenting LCs.3 Genetic and other factors, such as prior treatment with chemo-therapeutic agents, may also be involved in the pathogenesis of PLCH.1
Contributing Institution:
San Marco Veterinary Clinic and Laboratory
Pathology division
Viale dell’Industria 3, 35030
Veggiano (PD), Italy
https://www.clinicaveterinariasanmarco.it/
JPC Diagnosis:
Lung: Histiocytosis, alveolar, chronic, diffuse, severe, with fibrosis, type II pneumocyte hyperplasia, and smooth muscle hyperplasia.
JPC Comment:
This case of feline pulmonary Langerhans cell histiocytosis provided participants with an opportunity to review proliferative histiocytic disease in cats and complements the first case in this week’s conference, a case of canine systemic reactive histiocytosis.
As in dogs, proliferative histiocytic disease of cats generally arise from dendritic cells - either interstitial dendritic cells or epidermal dendritic cells (Langerhans cells). Feline pulmonary Langerhans cell histiocytosis is the only proliferative disease of Langerhans cells in cats, as a feline equivalent canine cutaneous histiocytoma has not been documented. Proliferative diseases of interstitial dendritic cells include feline progressive histiocytosis and histiocytic sarcoma (except the hemophagocytic form, which likely arises from macrophages).
Feline progressive histiocytosis and histiocytic sarcoma can both affect the lung and are histologic differentials for this section. FPH occurs in older cats and starts as single to multiple raised, alopecic, nonpainful and nonpruritic nodules in the skin. The lesions do not regress, but wax and wane, and in advanced cases, can spread to internal organs, such as the lungs, liver, and spleen.4,8,9 Histiocytic sarcoma can occur as a primary (localized) or metastatic (disseminated) neoplasm in the lung of cats, though it is less common in cats than in dogs.9 Histiocytes of both FPH and histiocytic sarcoma are expected to be negative for E-cadherin, which is normally expressed by Langerhans cells, and allows these differentials to be ruled out in this case.8,9
Langerhans cells were first discovered by German physician Paul Langerhans (1847-1888) while working in Rudolf Virchow’s laboratory at the Berlin Pathologic Institute. Langerhans was also the first to describe the pancreatic islets, now known as the islets of Langerhans, which he discovered nestled among pancreatic acini in the rabbit pancreas. Langerhans did not discover the function of these cells which bear his name; his academic career ended early after a pulmonary infection drove him to relocate to Portugal and return to medical practice. In this coastal location, he also developed a keen interest in marine biology, and several species of marine worms bear his name. Langerhans died when he was only 41 due to a severe kidney infection.11
References:
- Argenta FF, de Britto FC, Pereira PR, Rissi DR, Gomes C, da Costa FVA, Pavarini SP. Pulmonary Langerhans cell histiocytosis in cats and a literature review of feline histiocytic diseases. J Feline Med Surg. 2020 Apr;22(4):305-312.
- Busch MD, Reilly CM, Luff JA, Moore PF. Feline pulmonary Langerhans cell histiocytosis with multiorgan involvement. Vet Pathol. 2008 Nov;45(6):816-24.
- Casolaro MA, Bernaudin JF, Saltini C, et al. Accumulation of Langerhans’cells on the epithelial surface of the lower respiratory tract in normal subjects in association with cigarette smoking. Am Rev Respir Dis 1988; 137: 406-411.
- Caswell JL, Williams KJ. Respiratory System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA: Elsevier Ltd. 2016:498-499.
- Coste M, Prata D, Castiglioni V, et al. Feline progressive histiocytosis: a retrospective investigation of 26 cases and preliminary study of Ki67 as a prognostic marker. J Vet Diagn Invest. 2019;31(6):801-808.
- Hirabayashi M, Chambers JK, Sumi A, Harada K, Haritani M, Omachi T, Kobayashi T, Nakayama H, Uchida K. Immunophenotyping of Nonneoplastic and Neoplastic Histiocytes in Cats and Characterization of a Novel Cell Line Derived From Feline Progressive Histiocytosis. Vet Pathol. 2020 Nov;57(6):758-773.
- Lopez A, Martinson SA. Respiratory system, mediastinum and pleurae. In: Zachary JF ed. Pathologic Basis of Veterinary Disease, 6th Louis, MO: Elsevier; 2017:554.
- Mauldin EA, Peters-Kennedy J. Integumentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA: Elsevier Ltd. 2016:728-730.
- Moore PF. A review of histiocytic diseases of dogs and cats. Vet Pathol. 2014 Jan;51(1):167-84.
- Rissi DR, Brown CA, Gendron K, Good J, Lane S, Schmiedt CW. Pancreatic Langerhans cell histiocytosis in a cat. J Vet Diagn Invest. 2019 Nov;31(6):859-863.
- Sakula A. Paul Langerhans (1847-1888): a centenary tribute. J R Soc Med. 1988; 81(7): 414-415.
- Son NV, Uchida K, Thongtharb A, et al. Establishment of cell line and in vivo mouse model of canine Langerhans cell histiocytosis. Vet Comp Oncol. 2019; 17(3):345-353.
- Valli VEO, Kiupel M, Bienzle D. Hematopoietic system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 3. 6th ed. Louis, MO: Elsevier Limited; 2016:243.