WSC 22-23
Conference 7
CASE IV:
Signalment:
4 day-old, female, Belgian blue x Holstein, Bos Taurus, cattle/bovine.
History:
This calf was from a 100 head dairy farm with a few weeks history of diarrhea in neonatal calves; diarrheal episodes were also observed in a few older calves and heifers. As the diarrhea resolved within a few days with no or only supportive treatment, the cause was not thoroughly investigated, although coprological examinations revealed Cryptosporidium. However, as four diarrheic calves had died in the previous week, the veterinarian submitted an untreated dead calf to our diagnostic laboratory. The same herd had experienced Salmonella Dublin-associated disease 5 years before.
Gross Pathology:
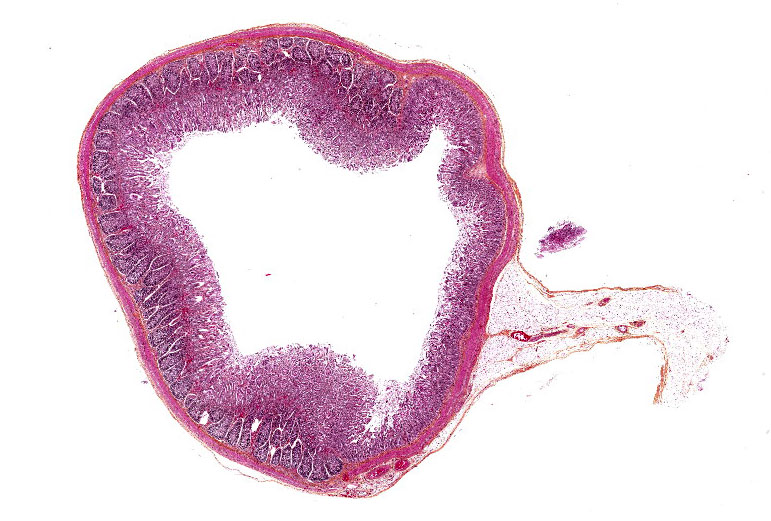
The calf was submitted partially frozen. Body condition was assessed as good. The perineal region was covered with abundant feces. There was marked bilateral enophtalmos and the subcutaneous tissues were “sticky”, consistent with dehydration. The thymus was moderately atrophied. Intestinal contents in the distal half of the small intestine, the cecum and spiral colon were not abundant, but they were liquid, pinkish to brownish with small clumps of fibrin-like material. The intestinal mucosa was hard to assess due to freezing/thawing, but there was no evidence of hyperemia, necrosis or fibrinous pseudomembranes. There was some milk in the rumen (“ruminal drinking”), but contents and mucosa of the gastric compartments were otherwise normal.
Laboratory Results:
Bacteriology: ileum and colon: ++++ non-hemolytic E. coli that were eae+, but F5-, STa-, Stx1- and Stx2- (3/3 colonies tested à EPEC). Negative for Salmonella. Virology: PCR negative for BCoV (coronavirus), type A rotavirus and BVDV. Parasitology: only a few Cryptosporidium oocysts were detected.
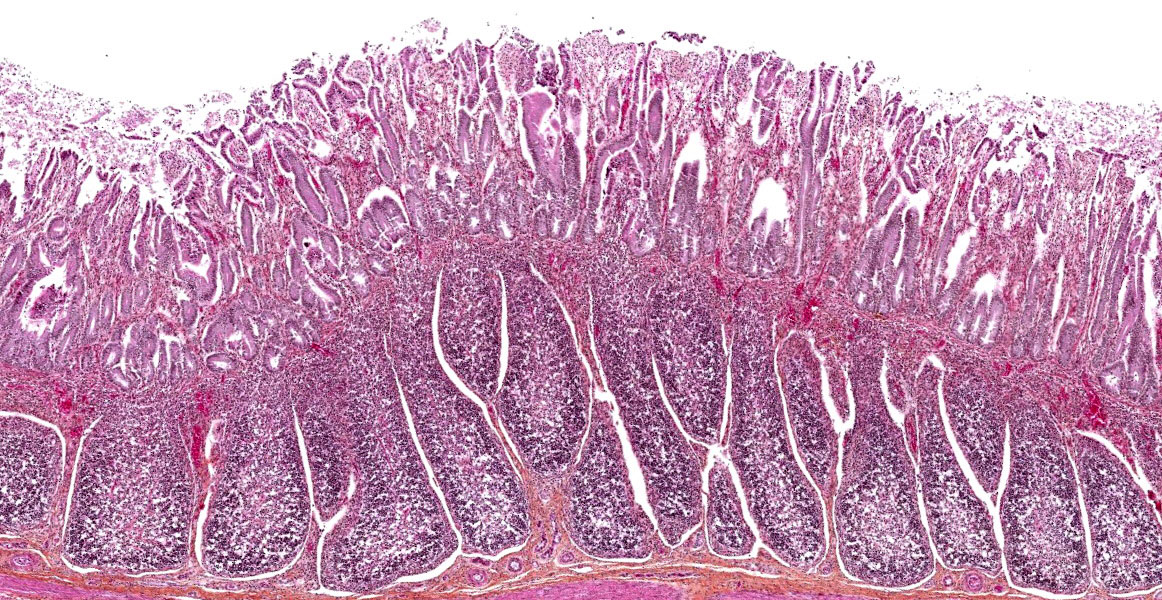
Microscopic Description:
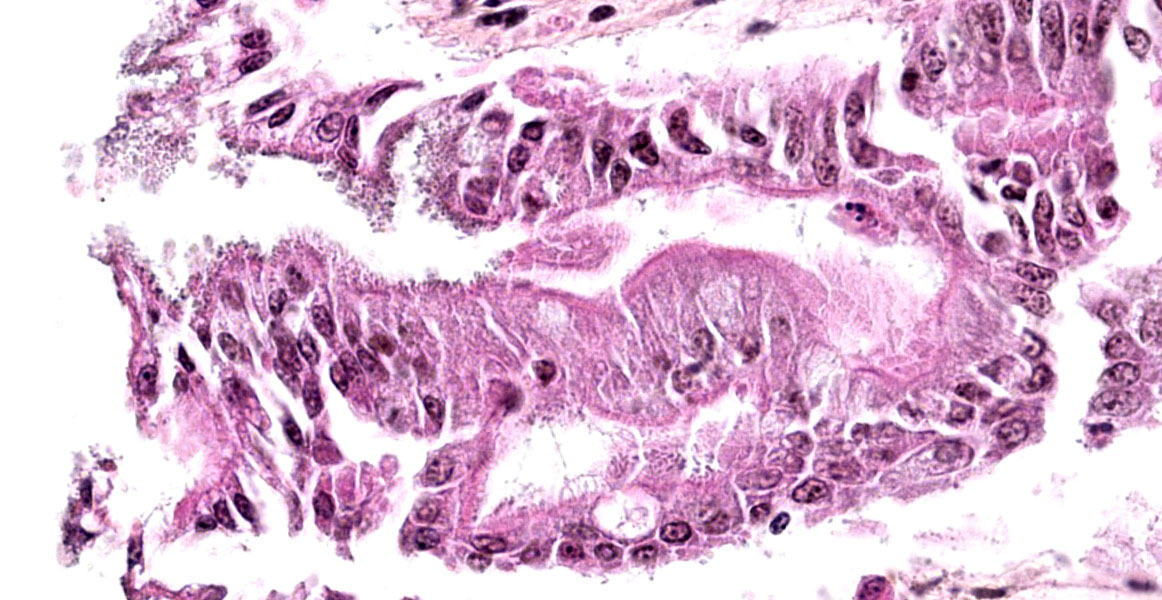
This is a section of ileum; there is variability among slides (either one of two sections) and moderate post-mortem changes, mostly desquamation. Multifocally, short bacilli are adherent to the villous enterocytes’ brush border, sometimes intimately with basophilia (bacteria) and palisading, and/or a scalloped epithelial surface. A Gram stain showed the bacilli to be Gram-negative (slide not provided). Similar lesions were present in the middle and distal jejunum and rarely in the colon. Associated changes vary from a few scattered luminal neutrophils to a luminal mass of fibrin, neutrophils and debris; in the latter case, there are a few capillaries with fibrin thrombi in the lamina propria. There is moderate lymphoid depletion in Peyer’s patches.
Contributor’s Morphologic Diagnoses:
Ileum, villous epithelium: intimately adherent bacilli with attaching and effacing morphology and minimal enteritis (consistent with EPEC infection)
Contributor’s Comment:
Based on the typical attaching and effacing intestinal lesions, the bacteriological results, and the absence of another cause, the diarrhea in this case was attributed to EPEC (enteropathogenic E. coli) infection, which is unusual as although EPEC infection is not infrequent in calves, it is rarely diagnosed as the main or sole cause of diarrhea. Although not conspicuous in examined sections, it was assumed that microulcerations were present and responsible for the fibrinosuppurative exudate seen grossly and in one of the sections. The Gram stain was done to be sure the bacteria were not enteroadherent cocci (e.g. Enterococcus durans), a rare cause of diarrhea in calves and piglets.
In humans, diarrheagenic E. coli (DEC) can be classified into six major classes, or pathotypes: enterotoxigenic E. coli (ETEC), enteropathogenic E.coli (EPEC), enterohemorrhagic E. coli (EHEC), enteroinvasive E.coli (EIEC), enteroaggregative E. coli (EAEC) and diffusely adherent E. coli (DAEC).1,4,9 Another pathotype, adherent invasive E.coli (AIEC), has been proposed to be involved in inflammatory bowel disease (Crohn’s disease; ulcerative colitis).1,7 Of these pathotypes, only ETEC is a significant cause of diarrhea in domestic animals, especially in piglets and calves,9,11 except in rabbits in which EPEC is a major cause of diarrhea;14 EPECs in humans primarily involves children, especially infants, in developing countries.4 Domestic animals, especially ruminants, are mostly reservoirs for EHECs, but EHECs can occasionally cause diarrhea in some species. The other pathotypes (EIEC, EAEC and DAEC) have not to our knowledge been reported in natural disease in domestic animals and will not be discussed further. ETECs, EPECs and EHECs are defined by virulence factors, have histopathologic characteristics (type and localization), and are associated with typical clinical signs; these categories are not mutually exclusive and some strains overlap pathotypes.4
ETECs are able to colonize the small intestinal villous enterocytes through one or more fimbriae and produce one or more enterotoxin that cause secretory diarrhea, which is watery and profuse.1,11 On light microscopy, bacilli are seen close to the apical portion of the villous enterocytes and are typically not associated with significant morphologic or inflammatory changes except in piglets in which mostly F4+ strains can occasionally cause a hemorrhagic gastroenteritis, possibly due to endotoxic shock.2,11
EPECs and the vast majority of EHECs cause a characteristic lesion known as “attaching and effacing” (AE) and are thus collectively known as attaching and effacing E. coli (AEEC).1, 9,11,12 AEEC lesions have described in humans/primates, rabbits, pigs, small ruminants, dogs, cats and birds (broiler chickens and turkeys).6,12This lesion is characterized at the light microscopic level by a colonization that is more intimate than for ETECs and associated with morphologic/degenerative changes in colonized enterocytes; bacteria in well-established lesions can be seen as coccobacilli, which are often more basophilic, in the brush border. Villous and cryptal epithelium in the small and/or large intestine can be involved. Colonized enterocytes eventually appear rounded-up, giving the epithelium a scalloped surface, and ultimately die and slough; if enterocyte loss is marked, villous atrophy/fusion, erosions and superficial ulcerations may be observed, with secondary inflammatory changes.1,11,12 Electron microscopic AEEC lesions are characterized by intimate attachment of the bacteria to the enterocytes’ apical cytoplasmic membrane, often with a cup-shaped actin “pedestal” in the underlying cytoplasm, and effacement of the microvilli.12 The genes responsible for the attaching and effacing lesion are located on the “locus of enterocyte attachment” (LEE), a large pathogenicity island; the LEE includes the eae (intimin) and, except in EHECs and “atypical” EPECs, bfp (bundle-forming pili) genes; detection of the eae gene is used for molecular identification of AEEC strains. The genesis of AE lesions essentially goes through four stages: 1) initial non-intimate attachment (bfp in typical EPECs; other fimbriae in atypical EPECs and in EHECs), 2) translocation of bacterial proteins, including Tir (translocated intimin receptor), into the enterocyte’s cytosol, 3) intimate attachment mediated by intimin, which binds to Tir, with underlying cytoskeletal reorganization (actin pedestal) and 4) invasion of bacteria into the cytoplasm (free or within vacuoles).4, 12 Attaching and effacing lesions are not restricted to AEEC. Citrobacter rodentium causes transmissible murine colonic hyperplasia in mice (one strain of Citrobacter rodentium had previously been called mouse-pathogenic E.coli), and Escherichia albertii is emerging as a new human diarrheagenic bacterium (including some misidentified as atypical EPECs) and avian pathogen; both produce AE lesions via the LEE genes.1,8,13
In contrast to EPECs, EHECs produce shigatoxins from either or both Stx1 and Stx2 families/groups; although EHECs have other potential virulence factors, this is the defining characteristic that separates EHECs from EPECs.1,4,9 EHECs are included in the shigatoxin-producing E. coli pathotype (STEC),1 and STEC and EHEC are sometimes used interchangeably, which is not correct as some STEC strains are neither AE nor diarrheagenic (e.g. Stx2e-producing in porcine edema disease).9 Some STEC strains are LEE-negative (and thus not AE), but produce hemorrhagic colitis and HUS, and are thus considered EHECs.1,9 Shigatoxins bind and cause damage to enterocytes and vascular endothelial cells (mostly intestinal and renal). Damage to renal endothelial cells is the basis of a thrombotic microangiopathy known as hemolytic uremic syndrome (HUS), which is a potentially lethal complication of EHEC infection in humans;1 HUS-like lesions have been reported in animals, but were not associated with EHEC-induced hemorrhagic colitis. EPEC-induced diarrhea in humans and other animals varies from mild to marked and may be variably blood-tinged if epithelial damage is extensive. The pathogenesis of EPEC-induced diarrhea does not involve toxin production and the exact mechanism is uncertain. Diarrhea is assumed to be due mainly to maldigestion (loss of brush border enzymes) and malabsorption (loss of absorptive surface), thus osmotic in nature, and dependent on lesion extent; alteration of water and ion channels due to the AE lesion may also be involved.1,11 EHEC-induced diarrhea in humans varies from mild, with only AE lesions, to severe with hemorrhagic colitis (or ileocolitis) and variably HUS; the best known and studied serotype associated with the latter is O157:H7.1 In other animals, EHEC-induced diarrhea has only been reported, to our knowledge, in calves and a goat.9,11,12 In calves, lesions are essentially similar to those in humans, but are generally less severe and not associated with HUS.9,11,12 This has been ascribed to the lack of Stx receptors in bovine endothelial cells; however, Stx does bind to bovine enterocytes and causes epithelial damage.9
The main causes of diarrhea in young calves are E. coli, type A rotavirus, bovine coronavirus, Cryptosporidium parvum and Salmonella enterica subsp. enterica (mainly serovars Typhimurium and Dublin).11 With regards to E. coli, the overwhelming majority of cases are due to F5+ Sta+ ETECs and are seen as a profuse watery diarrhea in the first 7-10 days of life.9,10 EPECs and EHECs have been isolated from feces of diarrheic and normal calves, usually 1-5 weeks of age (i.e. older than for ETECs);9,11 their importance in calf diarrhea is not clear. In autopsy cases, both EPECs and EHECs have been shown to cause diarrhea/enterocolitis in calves, but are much less frequently involved than ETECs and are often associated with other diarrheagenic agents. Clinically, the disease ranges from mild diarrhea to, mostly with EHECs, dysenteric (variably hemorrhagic). In our laboratory, EPECs is rarely diagnosed as the main or sole cause of diarrhea in calves and piglets, usually in immunocompromised animals.
Contributing Institution:
Faculty of veterinary medicine, Université de Montréal. Website: http://www.medvet.umontreal.ca
JPC Diagnosis:
Small intestine: Enterocyte degeneration, acute, multifocal, moderate, with villar blunting and numerous attached apical coccobacilli.
JPC Comment:
The contributor provides an outstanding explanation of attaching and effacing pathogens, E. coli classifications, and differential diagnoses for this case. At the heart of the attaching and effacing activity is a type 3 secretion system (T3SS) that allows the bacteria to inject virulence proteins (effectors) into the host cell cytoplasm. The T3SS is encoded in the LEE, as described by the contributor, which is highly conserved in all attaching-effacing pathogens.3 Also known as an injectisome, the T3SS contains a syringe shaped structure with a central channel which accommodates translocation of unfolded proteins, and each EPEC cell is estimated to have twelve needle complexes.3 Injection of effectors into the host cytosol enables the bacteria to modify host actin cytoskeleton, interfere with ion transport, and prevent formation of microtubules.3
Gram-negative bacteria have six dedicated secretion systems, numbered I through VI, which vary in structure and substances which can be transported. T1SS, found in bacteria such as Vibrio cholerae and Serratia marcescens, can transport substances across two membranes, the inner and outer bacterial membranes, in one step.5 Types IV and VI can transfer substances across three membranes: both bacterial membranes and one additional host membrane.5 T4SS is capable of transporting DNA and proteins, and is found in Brucella suis,Helicobacter pylori, and Neisseria gonorrheae, among others.5 T6SS, found in V. cholerea and Pseudomonas aeruginosa, may transfer effectors into host cells but may also be used to secrete substrates into the environment for bacterial communication and competition.5
T2SS and T5SS are isolated to the outer bacterial membrane. T2SS is found in most Gram-negative bacteria and transfers folded proteins from the periplasm to the extracellular space.5 T5SS is unique in that the substrates are auotransporters, forming their own channels in the outer membrane.5 This system requires proteins in the periplasm to be unfolded prior to secretion, and most of the substrates are virulence factors such as toxins or receptor-binding proteins.5 Examples include YadA from Yersinia enterocolitica and IcsA of Shigella flexneri.5 From assisting with adhesion to secreting toxic effectors, these secretion systems play important roles in pathogenesis of various Gram-negative infections.
References:
- Croxen MA, Law RJ, Scholz R, et al. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev. 2013;26(4):822-80.
- Faubert C, Drolet R. Hemorrhagic gastroenteritis caused by Escherichia coli in piglets: Clinical, pathological and microbiological findings.Can Vet J. 1992;33(4):251-6.
- Gaytan MO, Martinez-Santos VI, Soto E, Gonzalez-Pedrajo Bertha. Type Three Secretion system in Attaching and Effacing Pathogens. Front Cell Infect Microbiol. 2016; 6(129):1-25.
- Gomes TA, Elias WP, Scaletsky IC, et al. Diarrheagenic Escherichia coli. Braz J Microbiol. 2016; 47 (Suppl 1):3-30.
- Green ER, Mecsas J. Bacterial Secretion Systems - An overview. Microbiol Spectr. 4(1): 1-19.
- Janke BH, Francis DH, Collins JE, et al. Attaching and effacing Escherichia coli infections in calves, pigs, lambs, and dogs. J Vet Diagn Invest. 1989;1(1):6-11.
- Lee JG, Han DS, Jo SV, et al. Characteristics and pathogenic role of adherent-invasive Escherichia coli in inflammatory bowel disease: Potential impact on clinical outcomes.PLoS One. 2019;14(4): 1-14.
- Luperchio SA, Newman JV, Dangler CA, et al. Citrobacter rodentium, the causative agent of transmissible murine colonic hyperplasia, exhibits clonality: synonymy of C. rodentium and mouse-pathogenic Escherichia coli.J Clin Microbiol. 2000;38(12):4343-50.
- Moxley RA, Smith DR. Attaching-effacingEscherichia coli infections in cattle.Vet Clin North Am Food Anim Pract. 2010;26(1):29-56.
- Ngeleka M, Godson D, Vanier G, et al. Frequency of Escherichia colivirotypes in calf diarrhea and intestinal morphologic changes associated with these virotypes or other diarrheagenic pathogens. J Vet Diagn Invest. 2019;31(4):611-615.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary system.In: Jubb, Kennedy, and Palmer’s Pathology of domestic animals. Vol 2. 6th St.Louis, MO: Elsevier. 2016; 1-257.
- Wales AD, Woodward MJ, Pearson GR. Attaching-effacing bacteria in animals.J Comp Pathol. 2005;132(1):1-26.
- Yamamoto D, Hernandes RT, Liberatore AM, et al. Escherichia albertii, a novel human enteropathogen, colonizes rat enterocytes and translocates to extra-intestinal sites.PLoS One. 2017;12(2):1-16.