Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 4
18 September 2019
CASE I: 16-02795 (JPC 4087577)
Signalment: Adult (age and gender not specified) little red flying fox (Pteropus scapulatus).
History: This bat was in the care of a local wildlife rescue group, when its vocalisations became abnormal, then developed paresis which progressed to grand mal seizures. The bat was euthanased, and tissues submitted to the laboratory for exclusion of Australian Bat Lyssavirus (ABLV).
Gross Pathology: No gross lesions observed
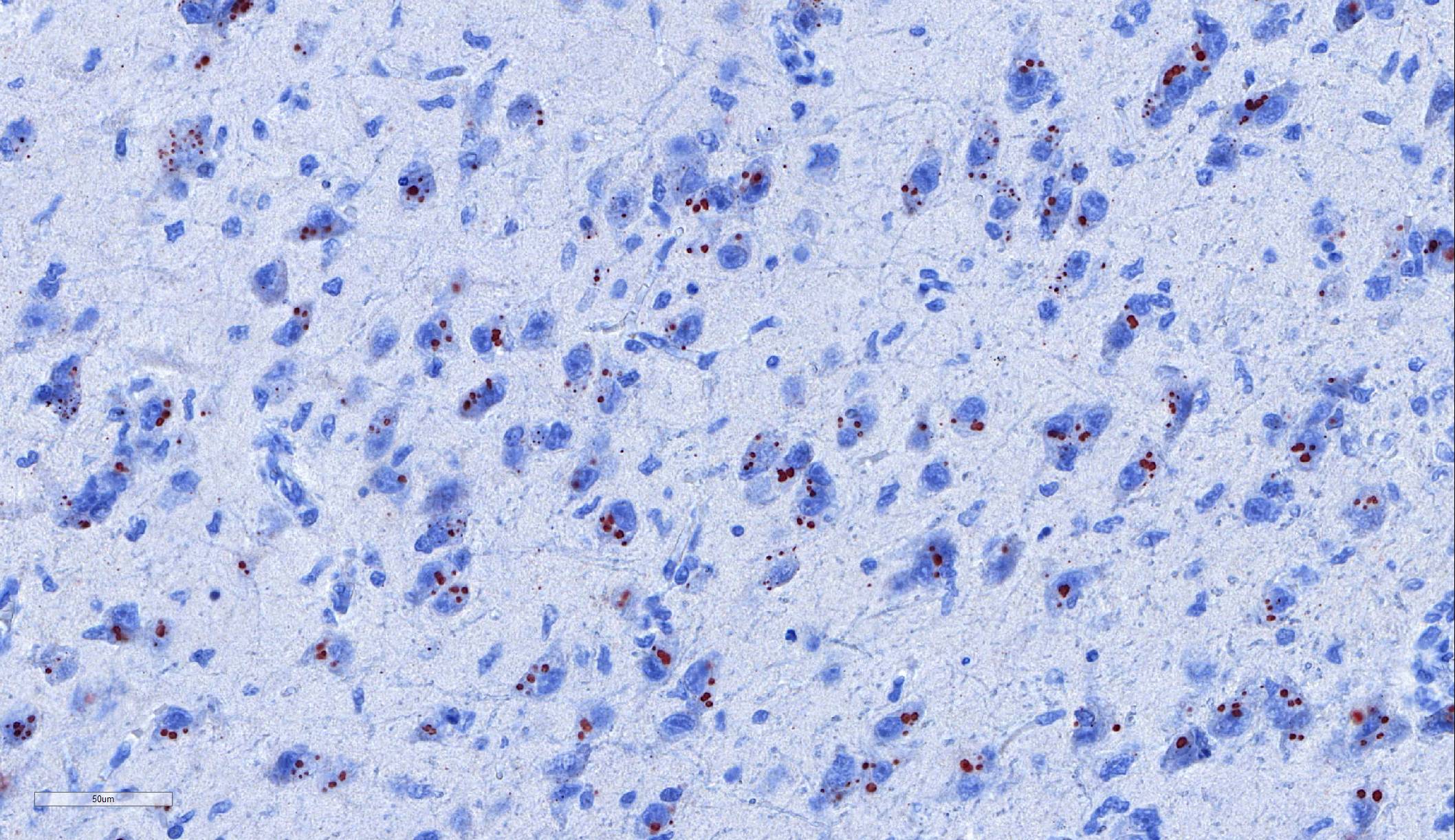
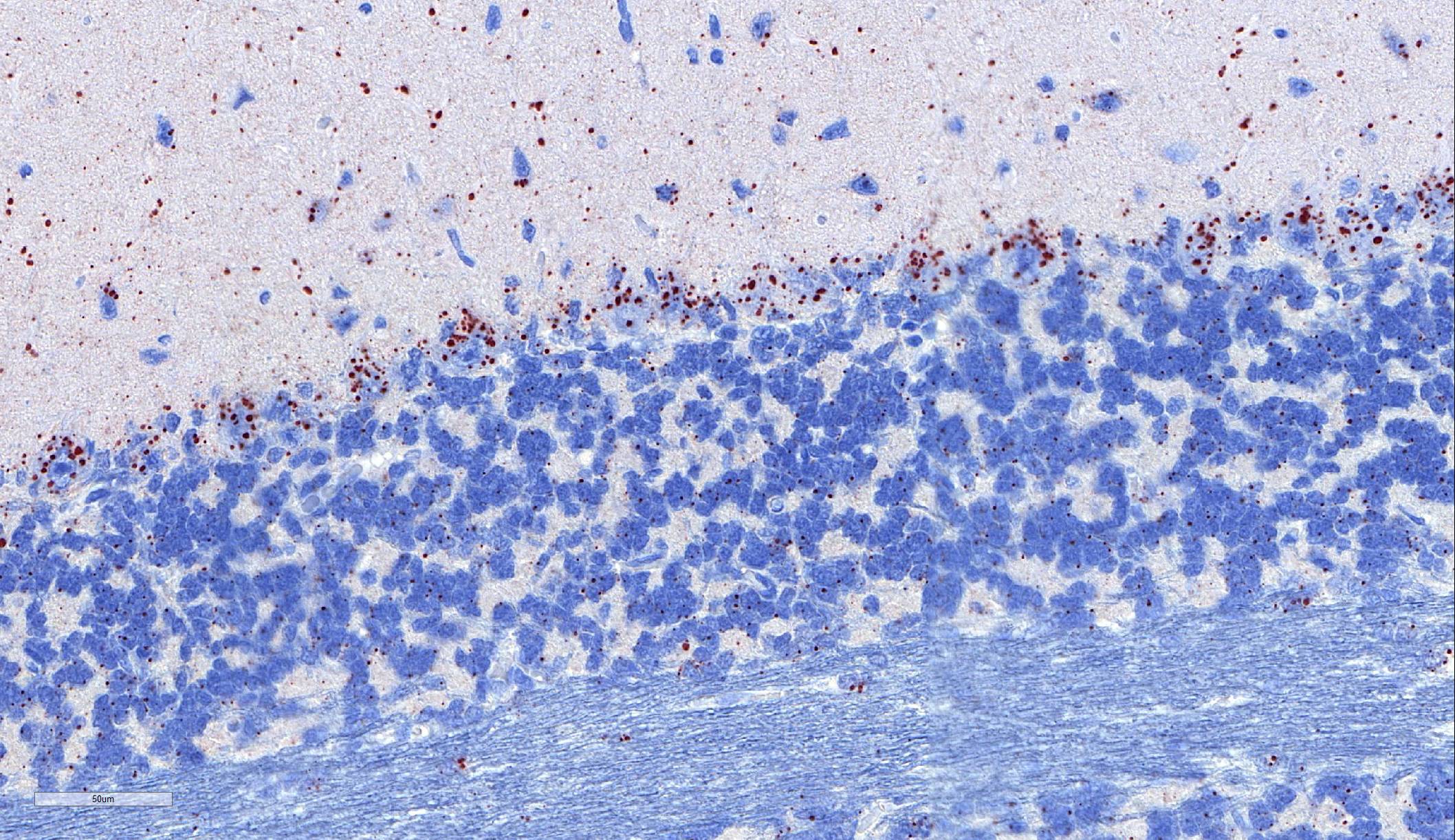
Laboratory results: Immunohistochemistry: Immunohistochemistry using monoclonal (HAM Mab 100) and polyclonal (Poly 663) antibodies raised against rabies nucleoprotein showed diffuse positive staining in neurons throughout the cerebral cortex and brainstem, and in cerebellar Purkinje fibres. ABLV antigen was most concentrated within neurons in the hippocampus, thalamus, and pons.
Microscopic Description:
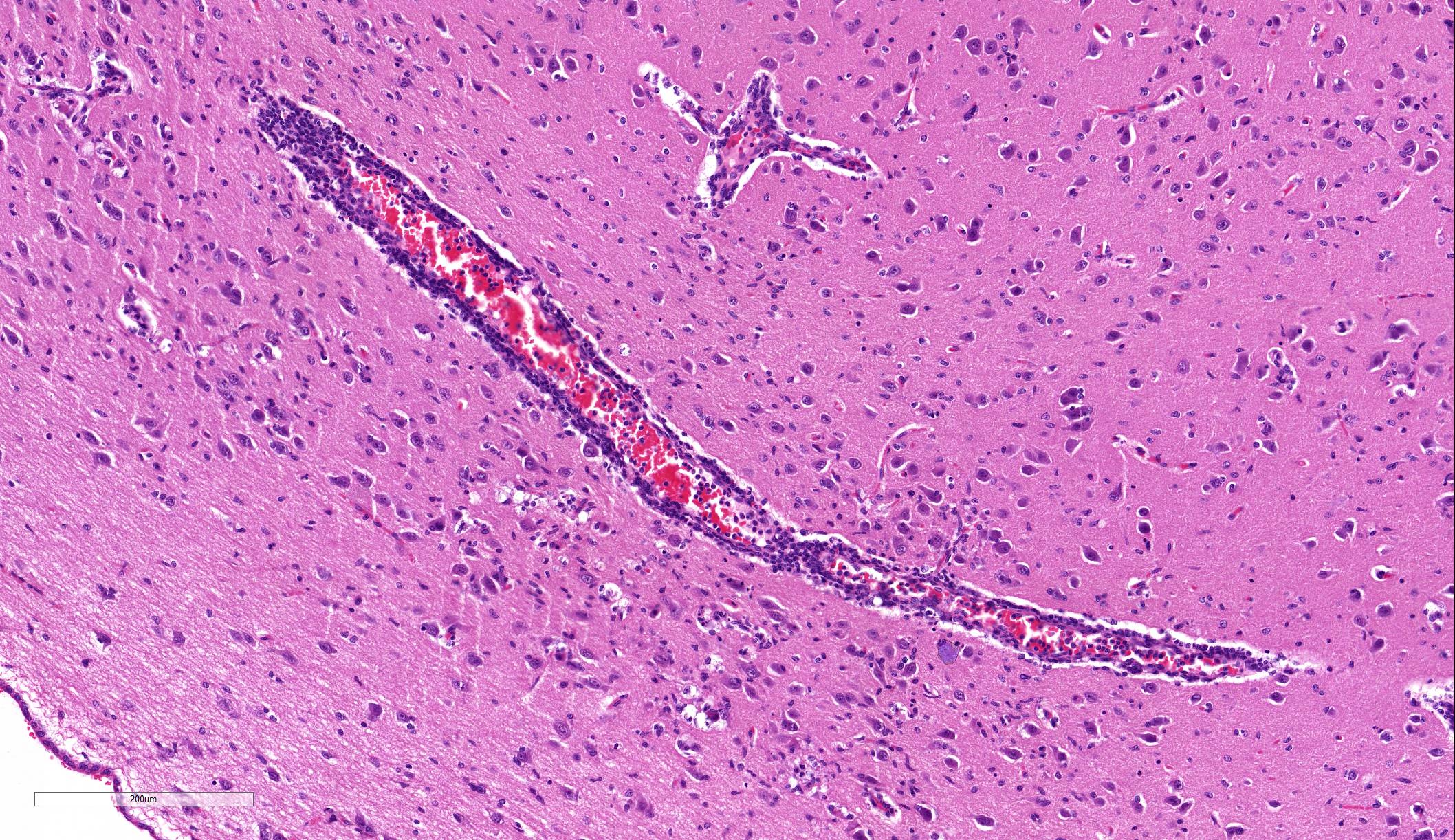
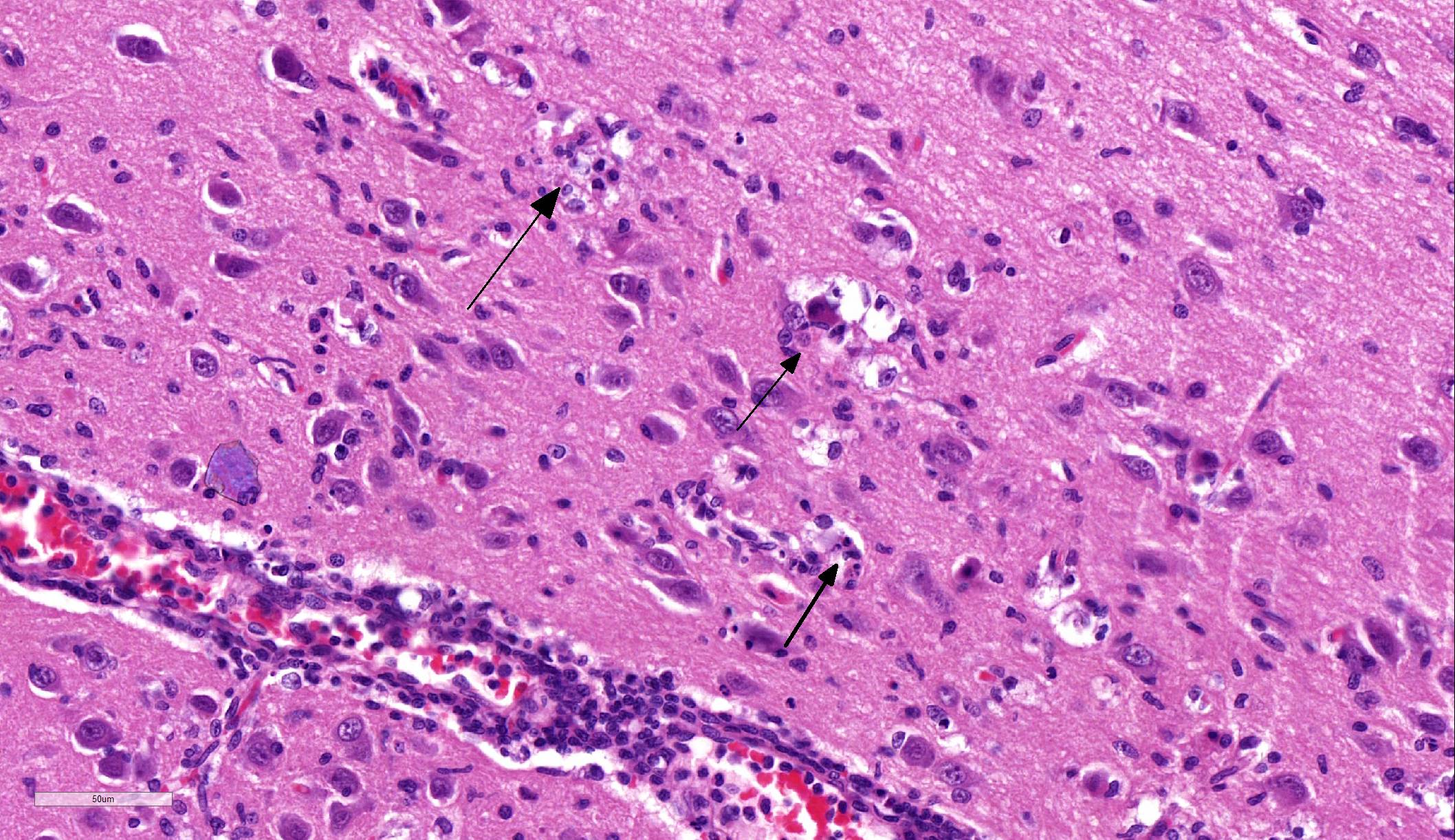
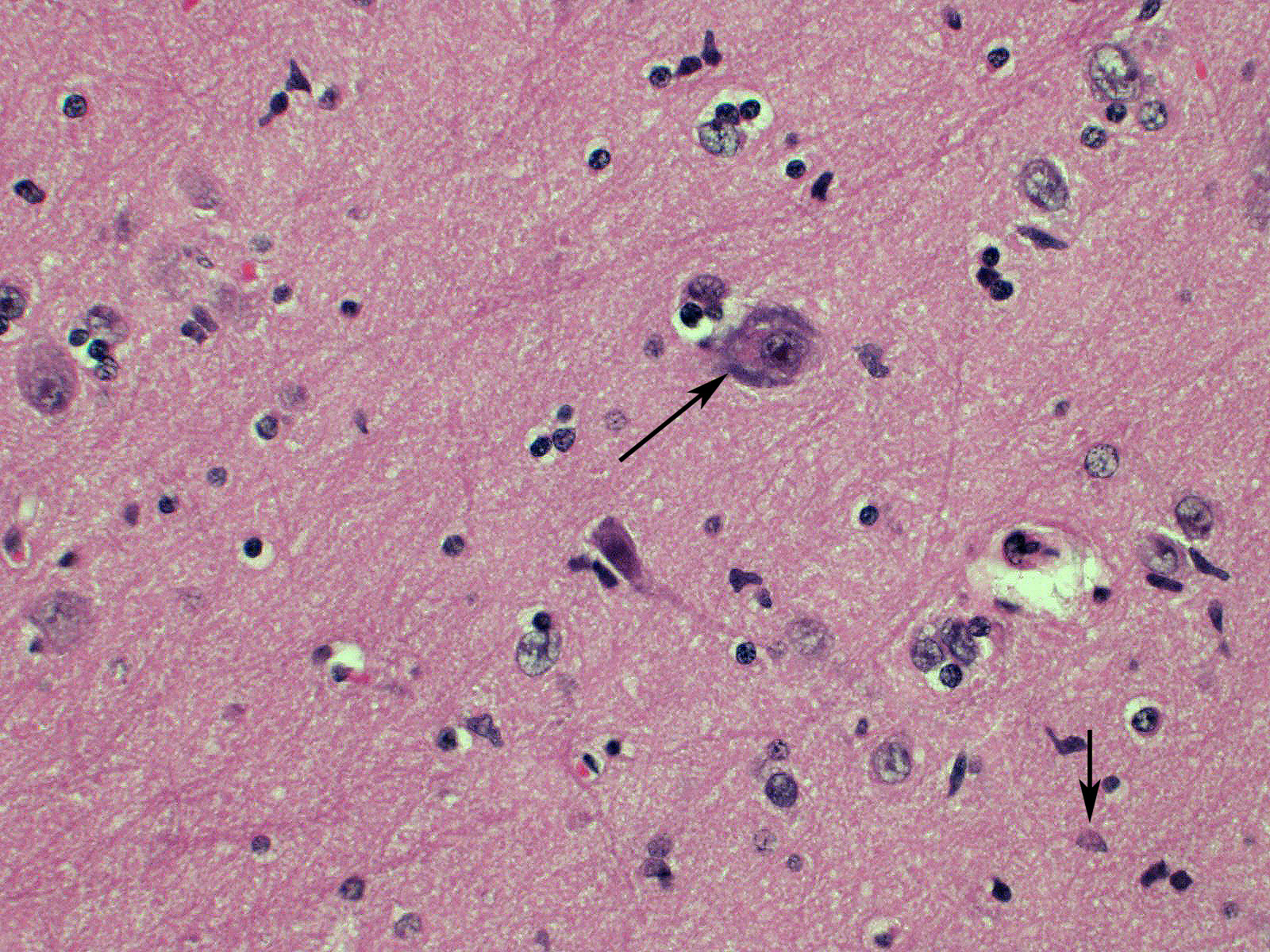
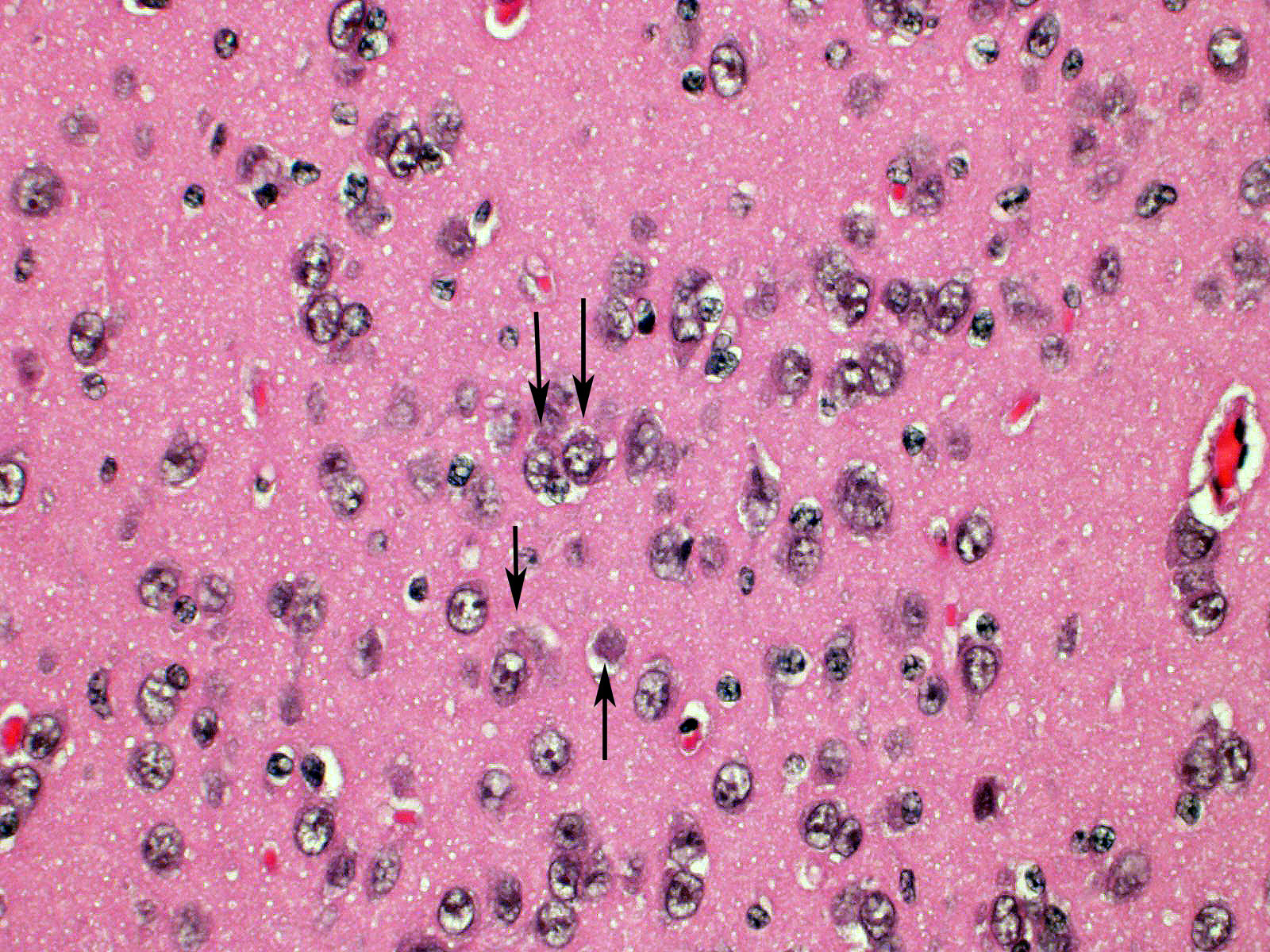
Brain: Blood vessels throughout the brainstem and cerebrum are surrounded by lymphocytes and fewer plasma cells. Multifocally within the brainstem, thalamus and rarely in cerebellar Purkinje cells, neurons have one to numerous oval, well demarcated, eosinophilic intracytoplasmic inclusions (Negri bodies). The cranial nerve is diffusely infiltrated with many lymphocytes and fewer plasma cells. Meninges in the brainstem are focally expanded with neutrophils and lymphocytes, with fewer plasma cells.
Contributor Morphologic Diagnosis:
1. Brain; brainstem, cerebrum, and cerebellum: Encephalitis, lymphoplasmacytic, subacute, diffuse, mild, with multifocal intraneuronal intracytoplasmic eosinophilic inclusions (Negri bodies)
2. Ganglion: Ganglioneuritis, lymphoplasmacytic, subacute, diffuse, moderate
3. Meninges: Meningitis, neutrophilic and lymphocytic, subacute, focal, mild, with multifocal calcification
Contributor Comment: Australian bat lyssavirus (ABLV) belongs to the genus Lyssavirus, family Rhabdoviridae, order Mononegavirales.10 The genus Lyssavirus includes seven genotypes: rabies virus (RABV, genotype 1), Lagos bat virus (genotype 2), Mokola virus (genotype 3), Duvenhage virus (genotype 4), European bat lyssavirus 1 (EBLV-1, genotype 5), European bat lyssavirus 2 (EBLV-2, genotype 6), and Australian bat lyssavirus (ABLV, genotype 7). ABLV is most closely related to classical rabies (genotype 1), and vaccination against rabies provides protection against exposure to ABLV. Two variants of ABLV have been described, in pteropid and insectivorous bats.5,6 Natural infections with ABLV have been described in horses,11 with affected animals displaying clinical signs similar to those of classical rabies. There have been three reported cases of ABLV infection in Australia, all of which were fatal.1,4,7,8
Microscopic lesions typically associated with Lyssavirus infections include a nonsuppurative encephalomyelitis, with multifocal gliosis, neuronal degeneration, and rare oval, eosinophilic intracytoplasmic viral inclusions (Negri bodies).2 The distribution of Negri bodies in domestic animals has been described as occurring most commonly in the hippocampus in carnivores, and in the Purkinje cells of herbivores.2 Hooper et al. (1999) published a survey of lesions in the brains of bats that had tested positive for ABLV, and reported lesions in the hippocampus, thalamus and midbrain, and medulla oblongata and pons.9 The authors observed Negri bodies in only 9 of 21 bats. Immunoperoxidase staining using antibodies raised against rabies viral nucleoprotein revealed a reaction pattern with a distribution similar to that of microscopic lesions. Positive immunohistochemical staining was noted to occur in the absence of lesions. Positive immunohistochemical staining was also reported in the dorsal root ganglia and in ganglia of the alimentary system. There was only loose relationship between staining of Negri bodies on H&E section and the presence of virus in neurons.
In the present case, the relationship between immunohistochemical staining and the presence of Negri bodies was similarly loose, with many more inclusions apparent after immunohistochemical staining. In addition, immunohistochemical staining of intraneuronal inclusions demonstrated a widespread distribution of viral antigen. Neuronal necrosis and gliosis were not features in the current case. This case was unusual in our laboratory with submission of only the fixed brain rather than the whole fresh bat and ABLV exclusion through tissue sampling of swabs from the oral cavity, salivary gland, and brain for PCR.11 Fresh salivary gland and brain tissues are subsequently sent to the national reference laboratory for direct fluorescent antibody testing, which is the international gold standard diagnostic test. While the results of immunohistochemical testing highlight the relative insensitivity of routine histology as a diagnostic method for Lyssaviruses it does demonstrate its ability to secure a positive diagnosis.
Bats are unique in that they are able to coexist with Lyssaviruses, as well as a number of other viruses that are lethal to most mammals (e.g. Hendra, Nipah, Severe acute respiratory system coronavirus [SARS-CoV], Ebola, Mekala, and Marburg viruses). Research indicates that the Australian black flying fox, Pteropus alecto, constitutively expresses interferon alpha genes,14 and type III interferons that are differentially expressed compared to type I interferons.13 However, the immunologic mechanisms by which bats are able to tolerate infection with these otherwise lethal viruses are only beginning to be unraveled.
Contributing Institution:
State Veterinary Diagnostic Laboratory
Elizabeth Macarthur Agricultural Institute
Woodbridge Rd
Menangle
NSW 2568
Australia
JPC Diagnosis: Brain:
Meningoencephalitis, diffuse, lymphoplasmacytic, mild with numerous neuronal
intracytoplasmic viral inclusions .
JPC Comment: The contributor has done an outstanding job describing Australian bat lyssavirus (ABLV) and the peculiarities noted upon comparison of histologic and immunohistochemical evaluation on tissue from infected animals. This case compares favorably with a series of ABLV-infected bats by Hooper et al.9 discussing the great variability of immunopositivity in neurons in tissues infected with lyssaviruses (similar to that in seen in rabies cases.) To maximize the diagnostic potential of immunohistochemistry in suspect cases of any lyssavirus, submission (and evaluation) of tissues in addition to cerebrum is highly recommended, to include spinal cord and dorsal root ganglia, adrenal gland, and gastrointestinal tract (inclusions may be found in GI nerve plexes.)9
As previously noted, ABLV infection in humans is a rare
finding, with only three reported cases, but with a mortality of 100%.
Infection rates in wild bat population have been estimated at less than 1%, but
purported to be markedly increased (5-10%) in sick or injured bats, the ones
most likely to come into contact with humans. Like rabies, direct contact is
required for zoonotic transmission, and in the documented cases of lyssavirus
in humans, the latent period ranged from one month to 27 months.4
Antemortem diagnosis of Australian bat lyssavirus in humans, as in rabies, is
difficult. Neuroimaging is usually of little benefit. CSF pleocytosis is mild
at best and present in only approximately 60% of cases.4 Lyssaviral
antibodies may appear late in the course of disease or not. The utility of
nuchal skin biopsy (considered to be 98% specific) is yet to be verified in
human cases of lyssavirus infection, but bat lyssavirus has been shown to
cross-react with rabies RT-PCR, so this test, as well as salivary PCR should be
considered.4 Clinically, the three cases of zoonotic bat lyssavirus
resembled the encephalitic or "furious" form of rabies, characterized by
hyperactivity, agitation, and spasms, especially of the face. As treatment for
lyssavirus has not been established, but post-exposure prophylaxis is
considered to be protective if offered.4
In addition to ABLV and rabies, two types of lyssavirus have been identified in European bats - European bat lyssavirus types 1 and 2 (EBLV-1 and EBLV-2); EBLV-1 is further subdivided into subtypes 1a and 1b. Both types have been reported as causing neurologic rabies-like syndromes in man, as well as cats and sheep.3
The distributed slides psed a challenge for participants as few identified lesions in the submitted tissue other than the numerous Negri bodies. The slide posted online at https://www.askjpc.org had an identifiable ara of meningeal inflammation, and the parenchyma in its proximity exhibited gliosis with rare neuronal necrosis and satellitosis (Fig 1-3). The architecture of one of the submitted sections was unfamiliar to the participants; many identified it as hippocampus until Dr. Andrew Cartoceti of the National Zoo identified it as olfactory cortex.
References:
1. Allworth A, Murray K, Morgan J: A human case of encephalitis due to a lyssavirus recently identified in fruit bats. Communicable diseases intelligence 1996:20:504-504.
2. Cantile C, Youssef S: Viral infections of the nervous system. In: Maxie G, ed. Jubb, Kennedy & Palmer's Pathology of Domestic Animals:. 6th ed.: Elsevier Health Sciences; 2016: 367-369.
3. Dacheau L, Larrous F, Mailles A et al. European bat lyssavirus transmission among cats, Europe. Emerg Inf Dis 2009; 15(2): 280-84.
4. Francis JR, Nourse C, Vaska VL, Calvert S, Northill JA, McCall B, et al.: Australian Bat Lyssavirus in a child: the first reported case. Pediatrics 2014:133(4):e1063-1067.
5. Gould AR, Hyatt AD, Lunt R, Kattenbelt JA, Hengstberger S, Blacksell SD: Characterisation of a novel lyssavirus isolated from Pteropid bats in Australia. Virus Res 1998:54(2):165-187.
6. Gould AR, Kattenbelt JA, Gumley SG, Lunt RA: Characterisation of an Australian bat lyssavirus variant isolated from an insectivorous bat. Virus Res 2002:89(1):1-28
7. Grattan-Smith P, O'regan W, Ellis P, O'fFaherty S, McIntyre P, Barnes C: Rabies. A second Australian case, with a long incubation period. Med J Austral 1992:156(9):651-654.
8. Hanna JN, Carney IK, Smith GA, Tannenberg AE, Deverill JE, Botha JA, et al.: Australian bat lyssavirus infection: a second human case, with a long incubation period. Med J Aust 2000:172(12):597-599.
9. Hooper PT, Fraser GC, Foster RA, Storie GJ: Histopathology and immunohistochemistry of bats infected by Australian bat lyssavirus. Australian Veterinary Journal 1999:77(9):595-599.
10. ICTV: Virus Taxonomy: 2015 Release.
11. Shinwari MW, Annand EJ, Driver L, Warrilow D, Harrower B, Allcock RJ, et al.: Australian bat lyssavirus infection in two horses. Vet Microbiol 2014:173(3-4):224-231.
12. Smith IL, Northill JA, Harrower BJ, Smith GA: Detection of Australian bat lyssavirus using a fluorogenic probe. Journal of Clinical Virology 2002:25(3):285-291.
13. Zhou P, Cowled C, Todd S, Crameri G, Virtue ER, Marsh GA, et al.: Type III IFNs in pteropid bats: differential expression patterns provide evidence for distinct roles in antiviral immunity. J Immunol 2011:186(5):3138-3147.
14. Zhou P, Tachedjian M, Wynne JW, Boyd V, Cui J, Smith I, et al.: Contraction of the type I IFN locus and unusual constitutive expression of IFN-alpha in bats. Proc Natl Acad Sci U S A 2016:113(10):2696-2701.