CASE IV: AK895 (JPC 4168135)
Signalment:
Approximately 7-year-old, female, rainbow trout (Oncorhynchus mykiss, Walbaum)
History:
The trout was reared in a concrete tank in a trout farming facility, provided with 10-12°C well water and fed bis in die with a commercial diet. A 9 cm x 7 cm mass was noted, bulging from the dorsal fin and altering its normal profile. Behavioral alterations were not present. The trout was euthanized with tricaine methanesulphonate overdose and underwent complete necropsy and histopathological examinations.
Gross Pathology:
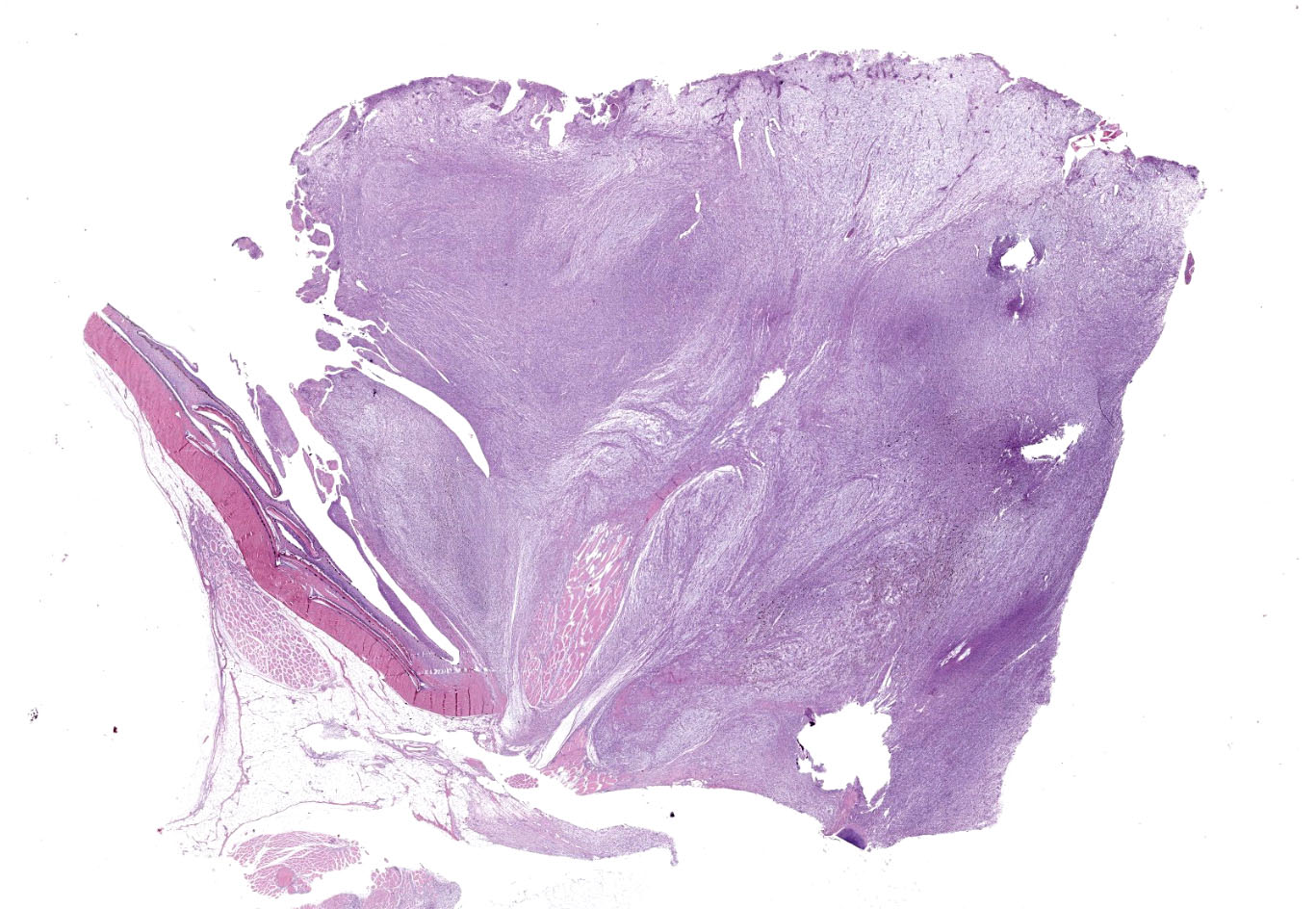
The mass was characterized by multinodular appearance, black to dark red discoloration with multifocal areas of ulceration, and soft consistence, with lardaceous appearance of the cut surface. When the coelomic cavity was opened, no alterations of the main internal organs were noted.
Laboratory Results:
None provided.
Microscopic Description:
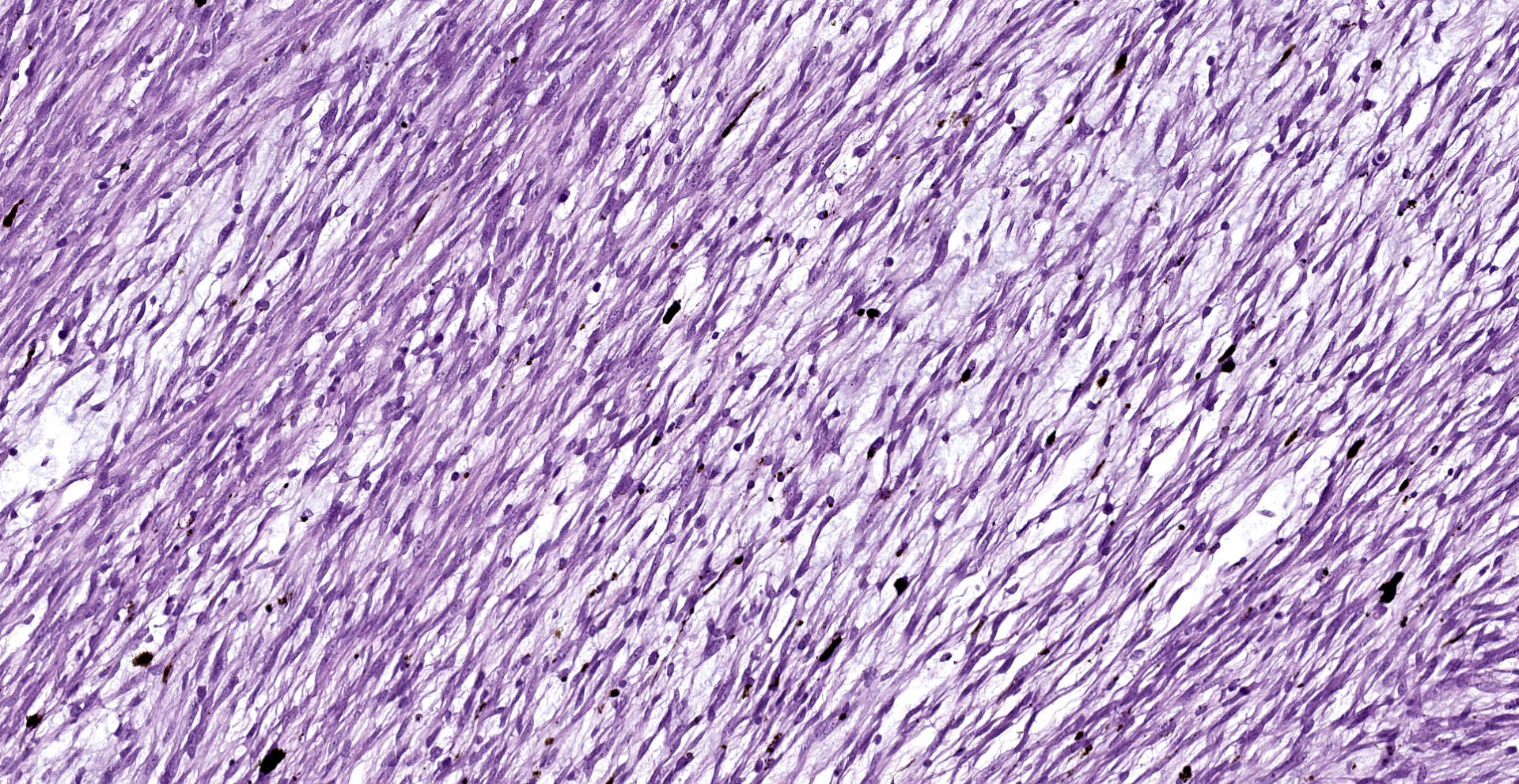
A multinodular neoplastic mass severely expands the dermis and the adjacent skin and extends to the cut borders. The mass is highly cellular, well-defined, non-encapsulated, and multifocally infiltrating the adjacent skeletal muscles. Neoplastic cells are haphazardly arranged in streams and fascicles, with occasional whorl formation. Cells are spindle-shaped and characterized by a scant fibrillary eosinophilic cytoplasm, a fusiform to wavy nucleus with basophilic finely stippled to vesicular chromatin, and up to 2 visible nucleoli. Cells are supported by scant fibrovascular stroma, associated to multifocal deposition of a loosely arranged clear matrix, with interspersed myriads of intensely basophilic granules (presumed proteoglycans). Neoplastic cells with multiple nuclei are rarely encountered. Anisocytosis and anisokaryosis are moderate, and mitotic figures are less than 1 per hpf. Numerous small and newly formed capillaries engorged by erythrocytes (neovascularization and congestion) are also visible, with occasional intravascular deposition of polymerized fibrillary eosinophilic material (fibrin thrombi) and erythrocyte extravasation (microhemorrhages). Small foci of inflammatory cells mainly composed of lymphocytes, plasma cells and, to a lesser extent, macrophages are also visible. At the periphery of the mass, small areas characterized by cellular hypereosinophilia and swelling, retention of cell borders, loss of cellular detail and nuclear pyknosis, loss of nuclei, and occasional faint eosinophilic nuclei or nuclear debris (karyorrhexis) (coagulative necrosis) are observed. Scattered melanophores are also noted throughout the mass.
Occasional filamentous bacteria elements are noticed and associated with the pocket scales and with small areas of epidermal ulceration. The neoplasia focally infiltrates the skeletal muscles causing sarcolemmal disruption, cytoplasmic flocculation, fragmentation, and hyalinization (muscle necrosis). This change is associated with infiltration of the myofibers by macrophages with foamy cytoplasm and occasional satellite cells (muscle regeneration) are evident. Intact small peripheral nerves are also noted at the edge of the mass.
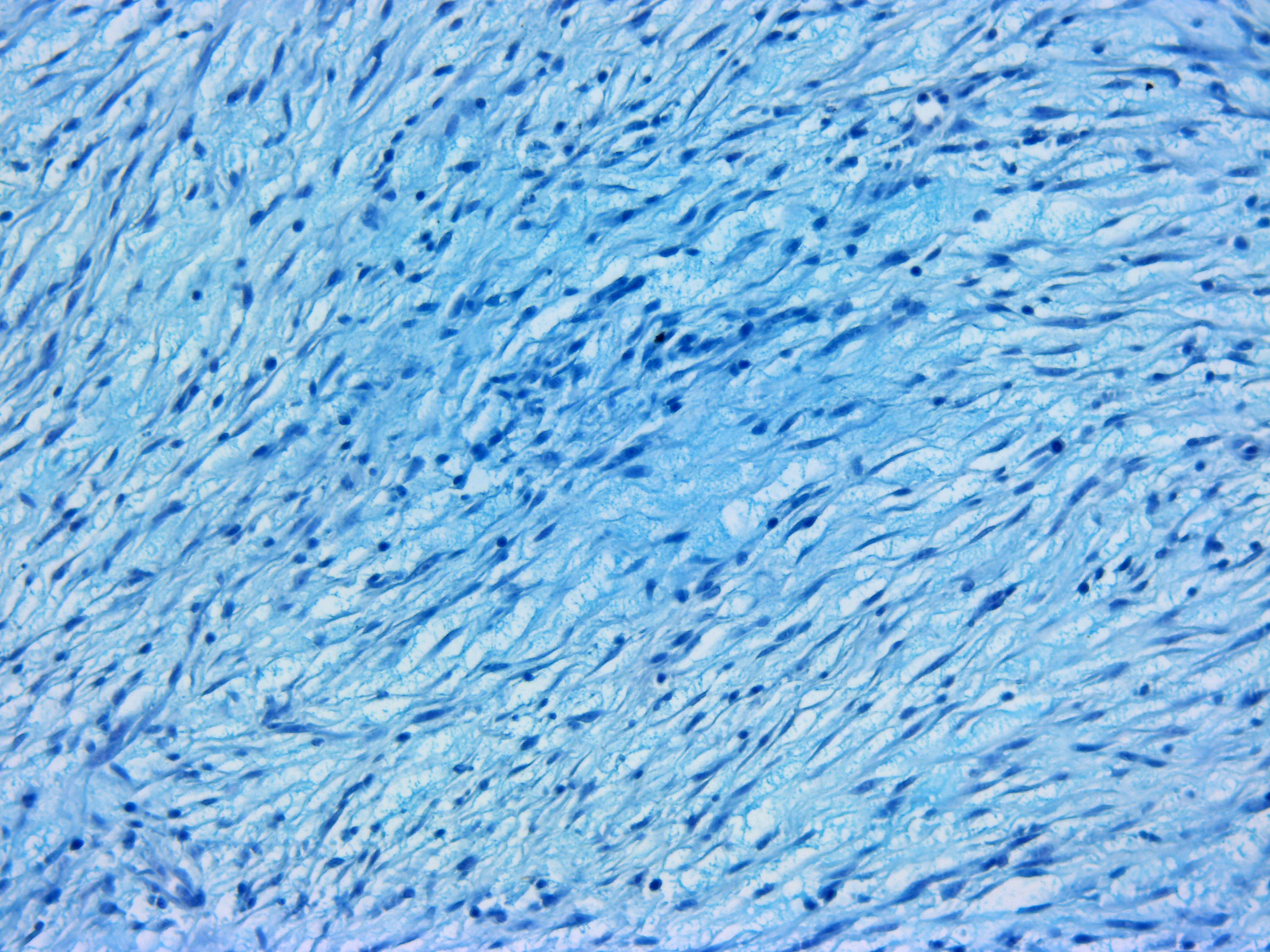
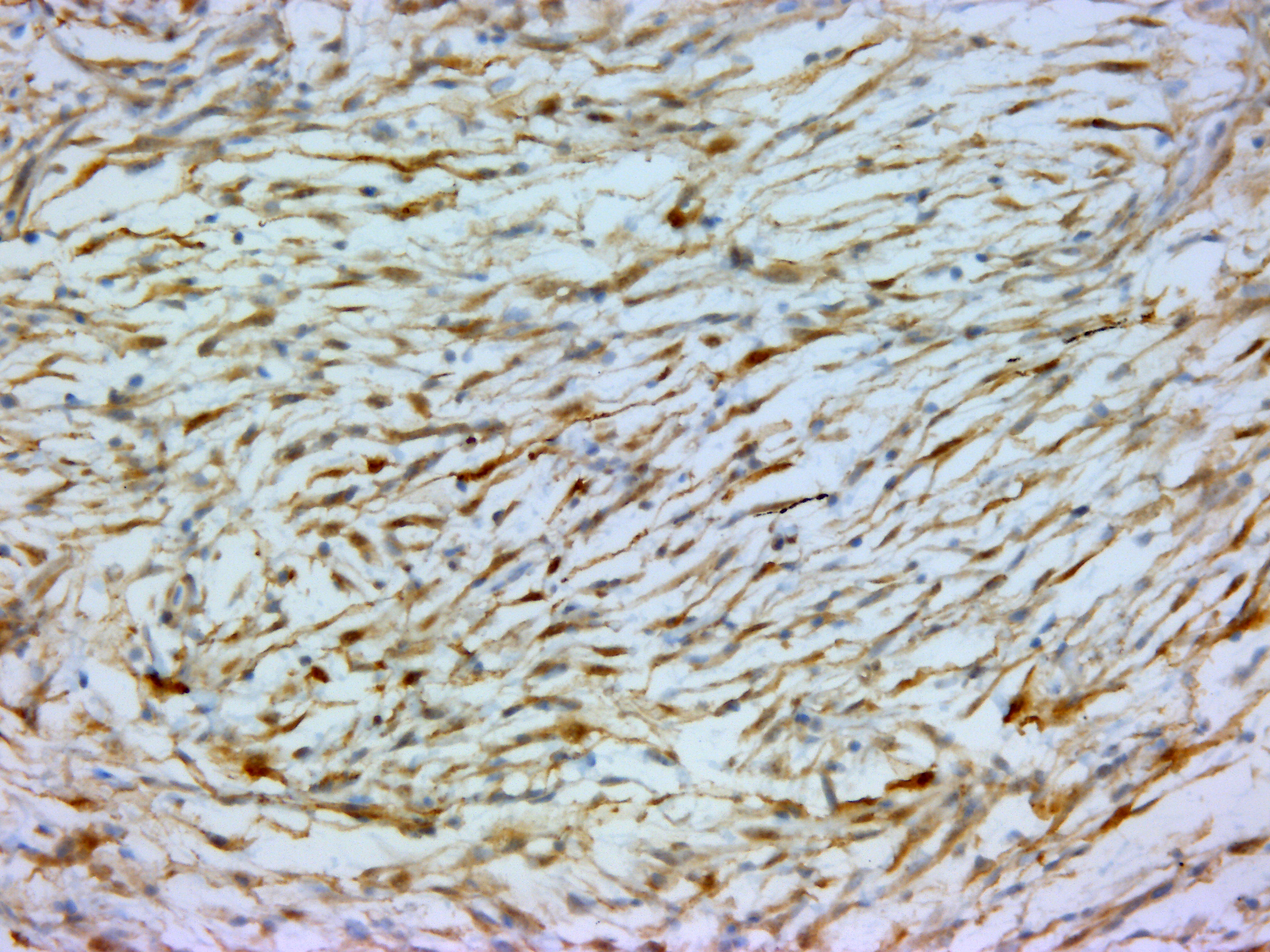
The mass was stained with Alcian-blue, Fontana-Masson, and immunohistochem-ically tested for S-100 expression. The Alcian-blue staining confirmed the mucinous origin of the extracellular matrix, while the Fontana-Masson staining yielded negative results, reasonably ruling out a melanocytic origin for this neoplasm. S-100 IHC specifically labeled neoplastic cells cytoplasm, reinforcing the hypothesis of a nervous origin.
Contributor?s Morphologic Diagnoses:
Rainbow trout (Oncorhynchus mykiss), dorsal fin: myxoid nerve sheath tumor (NST).
Contributor?s Comment:
Nerve Sheath Tumors (NSTs) were previously classified as peripheral nerve sheath tumors (PNSTs), but the term NST is now preferred given the complete lacking of an involvement of central nerves. In veterinary medicine, NSTs are classified as schwannoma, neurofibroma, perineurioma, and malignant nerve sheath tumor (MNST).
NSTs may arise in different body sites, predominantly in skin, spinal and cranial nerves, and autonomic ganglia of the heart, mediastinum, and thorax. In cats and dogs, NSTs are frequently characterized by the deposition of myxoid extracellular matrix.9 Similar to mammals, numerous piscine species can be affected by neoplasia, and some species, in particular, are predisposed to NST development. Among them are Goldfish (Carassius auratus)10, snappers (Lutjanidae)2,13, coho salmon (Oncorhynchus kisutch)6, damselfish (Pomacentrus partitus)11, and rainbow smelt (Osmerus mordax)7. In particular, schwannoma is the most common neoplasia in Goldfish3,10,12, and are reported also in seabream (Sparus aurata)4, and zebrafish (Danio rerio)5.
In humans, domestic animals, and Goldfish12, schwannomas show some characteristic patterns that can be of aid in the histopathological diagnosis. They frequently form cellular foci composed of in interlacing bundles of spindle cells (Antoni A areas) and hypocellular and less compact areas associated with loose myxoid material (Antoni B pattern).
When these two distinct histological patterns are missing several differentials need to be taken into consideration, including neurofibroma, perivascular wall tumors, sarcoid, myxoma/myxosarcoma, fibroma, melanoma, leiomyoma/leiomyosarcoma, low-grade MNST, ganglioneuroma and, in general, other soft tissue sarcomas.
IHC generally helps in such cases, and panels including S-100, NGFR, Olig-2, PRX, and SOX-10 may be applied for NST diagnosis. However, very few antibody clones have already been tested in fish, and among them, only the S-100 clone was available in our lab. The specific immunolabeling obtained for S-100 pointed out the nervous origin of the neoplastic cells, while the possible melanocytic origin was ruled out by the use of Fontana-Masson staining, which proved to be negative. This, together with the Alcian-blue positivity of the loosely arranged matrix (a feature which is not reported for melanocytic neoplasms), reinforced the diagnosis of a myxoid NST.
Contributing Institution:
Dept. Comparative Biomedicine and Food Science ? University of Padova
AGRIPOLIS ? Viale dell?Università 16
35020 Legnaro (PD) - Italy
JPC
Diagnosis:
Skin and dermis: Pigmented malignant neoplasm.
JPC Comment:
Nerve sheath tumors (and text suggestive thereof) are rarely described in ancient historical writings, likely attributable to their relatively low frequency of occurrence as well as an overall lack of anatomic understanding by ancient physicians. Hippocratic writings from 460-375 BC highlight this lack of understanding as ambiguous words are used to describe both nerves and tendons. Galen of Pergamon (129-200 AD) was one of the first to provide detailed descriptions of peripheral nerves extending from the spinal cord to the digits, largely based on dissections of pigs and apes.
Interestingly, Galen did not believe peripheral nerves were capable of repair following transection and a wounded nerve connected to the brain would result in convulsions. Galen recommended a salve composed of ground earthworms to treat nerve injury rather than surgical incision.9
The notion peripheral nerves are incapable of regeneration remained unchallenged until Cruickshank?s experiment investigating canine survival following vagal nerve transection in 1776. Cruickshank found dogs survived following unilateral transection vagal nerve but died when the contralateral nerve was incised in two weeks later. While examining the site of the first transected nerve, Cruickshank noted the presence of a white substance inconsistent with scar tissue and postulated it was regenerating nerve tissue. In subsequent experiments, he found dogs survived when the time interval between transections was icreased. Despite
this convincing evidence of nerve regeneration, the Royal Society rejected his thesis for publication based on over 1700 years of belief to the contrary. Haighton took the experiment one-step further 20 years later, transecting the initially transected vagal nerve shortly after the contralateral nerve, resulting in the death of the animal. Cruickshank and Haighton?s manuscripts were subsequently published in 1795.9
Odier of Geneva first used the term ?neurom? to describe tumors formed by diseased enlarged nerves in 1811. Over the ensuing decades, authors applied this term to describe both traumatic neuromas (as likely observed by Cruickshank) as well as both primary and metastatic tumors involving nerves. In 1864, Virchow separated peripheral nerve tumors of into ?true? and ?false? neuromas, with the former containing both nerve fibers and nerve sheath elements while the latter only contained elements of the nerve sheath (now known as nerve sheath tumors).9
Significant debate ensued over the next century about the cell of origin with Genersich first proposing ?false? neuromas (i.e. nerve sheath tumors) arose from Schwann cells while others argued they were composed of endoneurium. Harkin and Reed expanded the classification of peripheral nerve sheath tumors in 1969 by identifying schwannomas and neurofibromas as separate entities. Their use of electron microscopy confirmed of Schwann cell involvement in schwannomas but they were unable to determine the neurofibroma cell of orgin.9
Subsequent advances in the fields of molecular and cellular biology have generated substantial evidence that both schwannomas and neurofibromas originate from Schwann cells lacking expression of tumor suppressor gene known as NF1. This gene is responsible for the production of NF1 protein, which inactivates Ras by promoting the autohydrolysis of GTP to GDP. Notably, only Schwann cells in schwannomas are deficient in NF1 gene expression whereas all the supporting cells in neurofibromas are NF1 deficient with Ras hyperactivity, with an increased rate of proliferation in response to growth factors released from tumorigenic Schwann cells.9 As noted by the contributor, additional nerve sheath tumors including perineurioma, and malignant nerve sheath tumor have also been described.8
Neoplasia may present in fish in a similar manner as in mammals, with reports of metastatic disease. Farmed species are not as commonly affected as others, largely as the result of inherently prohibitive rapid production cycles that reduce the incidence neoplasia development in those populations as a whole. However, older fish (as in this case) are also encountered in hatcheries, such as when utilized as broodstock and are more likely to present with neoplasia. Compared to farmed species, the rate of detection is much higher in ornamental and wild species with longer lifespans. Neoplasia is most commonly associated with highly proliferative tissues such as the skin, gills, liver, gastrointestinal tract, in addition to numerous reports of nerve tumors as previously noted by the contributor.1
In this particular case, additional sections submitted to the WSC underwent a battery of additional stains including vimentin, Sox10, OLIG2, muscle specific actin, and Fontana-Masson; however, these stains and their associated procedures have not been verified in fish. The neoplastic cells demonstrate diffuse, moderately strong nuclear and cytoplasmic immunoreactivity for S100, indicating the cells are of neural crest origin. Neoplastic cells are immunonegative for muscle specific actin. Vimentin, Sox10, OLIG2, and Fontana-Masson are non-contributory. In addition, participants noted approximately 10% of the cells within the neoplasm have cytoplasmic melanin, which generated additional discussion in regard to a differential diagnosis of a melanocytic neoplasm, which would also be expected to be immunoreactive for S100. Unfortunately the additional stains required to make this differentiation were non-contributory in this case. Given an inability to definitively identify this neoplasm via histomorphologic characteristics nor available histochemical and immunohistochemical stains, participants preferred a general diagnosis of pigmented malignant neoplasm.
References:
1. Brocca G, Zamparo S, Quaglio F, Verin R. Metastatic myxoid nerve sheath tumor of the dorsal fin in a rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis. 2021;44(11):1875-1878.
2. Lucké, B., 1942. Tumours of the nerve sheaths in fish of the snapper family (Lutianidae). Archives of Pathology, 34, 133?150.
3. Marino, F., Germanà, A., Bambir, S., Helgason, S., De Vico, G., Macrì, B., 2007. Calretinin and S-100 expression in goldfish, Carassius auratus (L.), schwannoma. Journal of Fish Diseases, 30, 251-253. doi: 10.1111/j.1365-2761.2007.00776.x. PMID: 17394528.
4. Marino, F., Germanà, A., Panebianco, A., 2008. A case of schwannoma in farmed seabream Sparus aurata. Diseases of Aquatic Organisms, 82, 249-252. doi: 10.3354/dao01992. PMID: 19244977.
5. Marino, F., Lanteri, G., Rapisarda, G., Perillo, A., Macrì, B., 2012. Spontaneous schwannoma in zebrafish, Danio rerio (Hamilton). Journal of Fish Diseases, 35, 239-242. doi: 10.1111/j.1365-2761.2011.01335.x. PMID: 22324347.
6. Masahito, P., Ishikawa, T., Yanagisawa, A., Sugano, H., Ikeda, K., 1985. Neurogenic tumors in coho salmon (Oncorhynchus kisutch) reared in well water in Japan. Journal of the National Cancer Institute, 75, 779?790. https://doi.org/10.1093/jnci/75.4.779
7. Morrison, C.M., Harshbarger, J.C., McGladdery, S.E., 1993. Schwannoma in rainbow smelt. Journal of Aquatic Animal Health, 5, 317?323.
8. Powers CJ, Friedman AH. A brief history of surgery for peripheral nerve sheath tumors. Neurosurg Focus. 2007;22(6):E1.
9. Roccabianca, P., Schulman, F.Y., Avallone, G., Foster, R.A., Scruggs, J.L., Dittmer, K., Kiupel, M., 2020. Volume 3: Tumors of Soft Tissue. Surgical Pathology of Tumors of Domestic Animals. M. Kiupel (Ed.) (pp. 232-239). Gurnee, IL: Davis-Thompson DVM Foundation.
10. Schlumberger, H.G., 1952. Nerve sheath tumors in an isolated goldfish population. Cancer Research, 12, 890?899.
11. Schmale, M.C., Gibbs P.D.L., Campbell C.E., 2002. A virus- like agent associated with neurofibromatosis in damselfish. Diseases of Aquatic Organisms, 49, 107?115. doi: 10.3354/dao049107
12. Sirri, R., Diana, A., Scarpa, F., Brachelente, C., Vitellozzi, G., Ceredi, L., Mandrioli, L., 2015. Ultrasonographic and pathologic study of schwannoma in a Goldfish (Carassius auratus). Veterinary Clinical Pathology, 44, 586-591. doi: 10.1111/vcp.12285.
13. Williams, E.H. Jr, Rand, T.G., Bunkley-Williams, L., 2000. Neurofibromas in Gray Snappers, Lutjanus Griseus, from Bermuda and the unusual distribution of Nerve Sheath Tumors in Snappers at the Northern Extremes of West Indian Waters. Caribbean Journal of Science, 3-4, 344?346.