Signalment:
Gross Description:
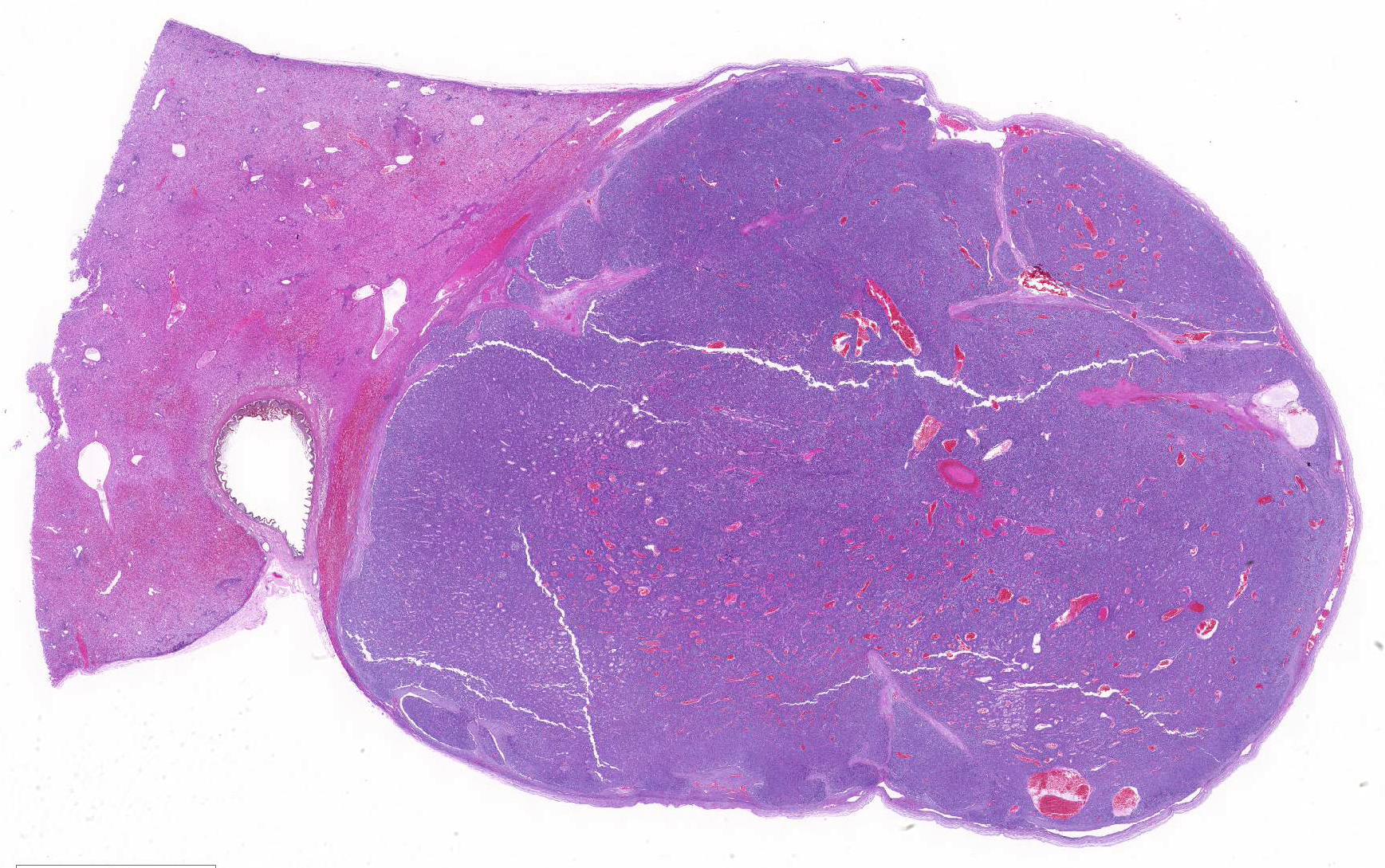
At necropsy and examination of visceral organs, expanding the right border of the right median liver lobe, compressing hepatic parenchyma and adjacent gallbladder was a 4cm x 1.7cm x 2.1cm, white to light brown nodular mass. Nodules of this neoplasm were surrounded by fibrovascular tissue, and the neoplasm was well-demarcated and encapsulated.
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Hepatoblastoma is a rare tumor in domestic animals and have been reported in the dog, alpaca, horse, mouse, and cat. 6,8,9,10,11,12,13,14 While other induced and naturally occurring hepatic tumors have been reported in cynomolgus macaques, hepatoblastoma is a tumor that has not been reported in literature from this species.
Hepatoblastomas and their subtypes in humans can be further characterized by immunohistochemistry markers such as alpha fetoprotein (AFP), glypican 3 (GPC3), carbamoyl phosphate synthetase 1 (CSP1), vimentin, cytokeratin and beta-catenin. 2, 7 Classification of the hepatic tumor in this fetal macaque tumor was attempted based on the neoplastic cell morphology and immunohistochemistry results using the recently published classification for pediatric liver tumor by López-Terrada D.2
AFP is expressed in normal, non-neoplastic human fetal liver and is also seen in human liver tumors and some germ cell tumors. In this study, AFP was weakly expressed by hepatocytes of liver parenchyma adjacent to the hepatoblastoma, while strong positive AFP immunolabelling by neoplastic cells of the hepatoblastoma highlighted architecture of the neoplastic embryonal hepatocytes and supported diagnosis of embryonal subtype of hepatoblastoma for this fetuss liver tumor. As AFP is also a secreted protein, increased background and serum labeling of AFP could also be seen in immuno-histochemically labeled sections, 7 and was also noted in blood vessels of AFP labeled sections of this fetal macaques tumor. While AFP is currently the most reliable and consistent marker in hepatoblastoma,4 approximately 5-10% of human hepato-blastomas are negative for AFP and other markers such as Delta-like homolog (DLK1) are being investigated as potential serum markers for diagnosis of this tumor.15
Pancytokeratin expression in human hepatoblastomas can be variable while vimentin is negative on the epithelial components.7 Cytokeratin 18 (CK18) is expressed in adult epithelial tissues such as liver, lung, kidney, pancreas, gastrointestinal tract, mammary glands and their associated tumors, and an increased CK18 expression in several cancer types of human patient is linked to poorer prognosis.16 In this study, the strong positive pancytokeratin and CK18 expression by the fetal hepatic neoplasm are consistent with hepatocyte lineage of the neoplastic cells. The negative and much smaller tumor area of vimentin labeling on the mesenchymal components of the liver tumor, in this case, indicate a hepatoblastoma with predominant epithelial cell component.
Hepatoblastomas in both humans and mouse have high prevalence of deletion mutation in GSK-3β binding region of β-catenin gene of Wnt signalling pathway, with approximately 80-90% of hepatoblastomas in humans having mutations in beta-catenin (CTNNB1).2, 7, 17 The nuclear and/or cytoplasmic pattern of expression of β-catenin could be used to distinguish between neoplastic hepatic and biliary hepatocytes. 2, 7 Specifically, non-neoplastic hepatocytes and biliary epithelium have only distinct membranous beta-catenin labeling, while cytoplasmic expression of beta-catenin indicates the epithelial cells of neoplastic nature.2, 7
Neoplastic cells of the liver, in this case, have cytoplasmic beta-catenin immuno-labelling. Based on histopathology findings and positive pancytokeratin (MNF116), cytokeratin 18, alpha-fetoprotein, as well as the cytoplasmic beta-catenin immuno-histochemistry results, current findings are indicative of an embryonal subtype of hepatoblastoma in the liver of this macaque fetus.
JPC Diagnosis:
Conference Comment:
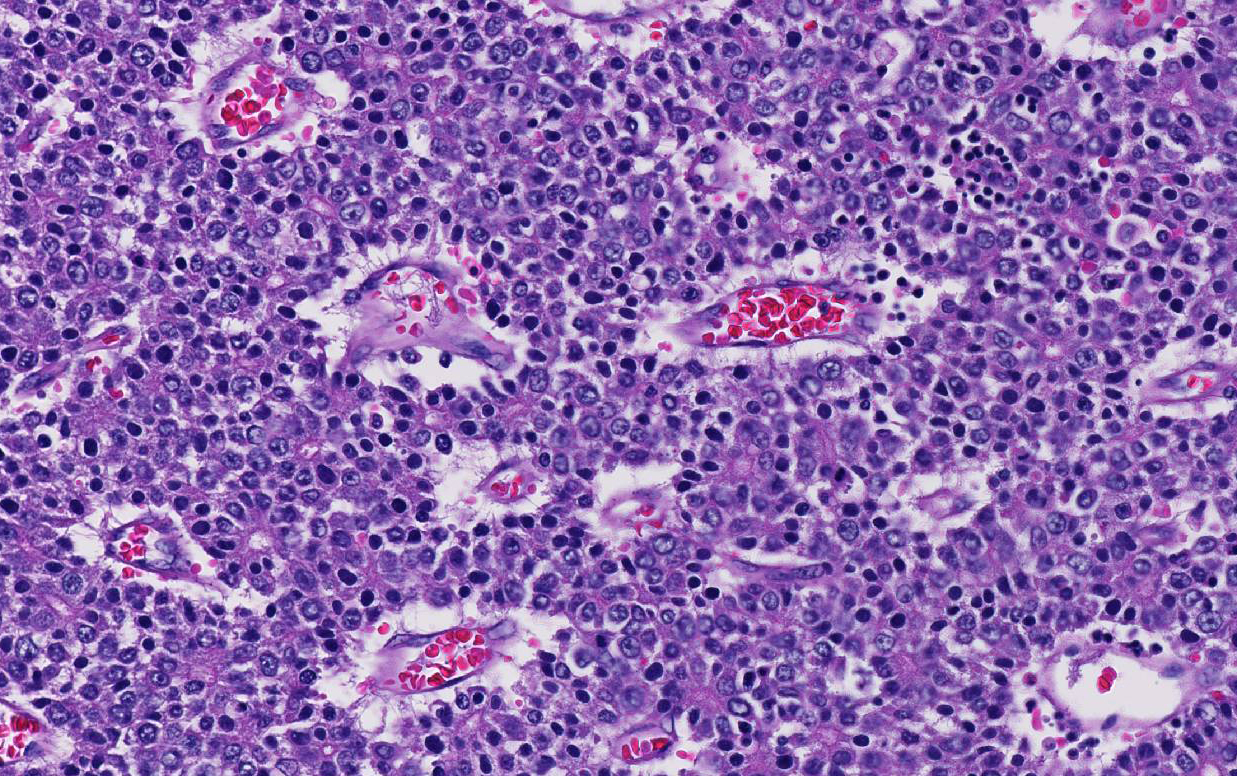
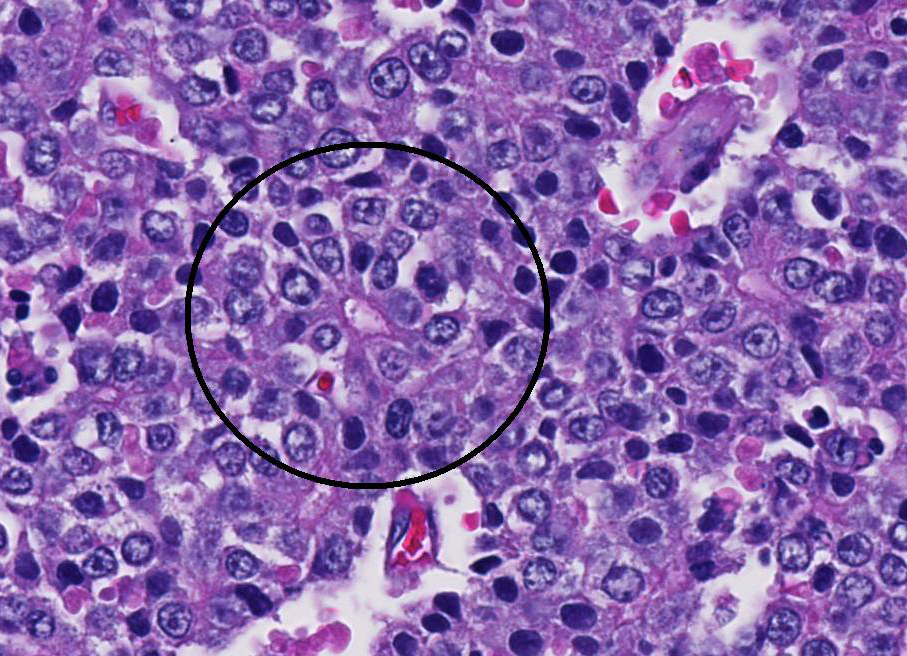
Conference participants noted that there is a marked increase in extramedullary hematopoiesis (EMH) as characterized by erythroid precursor cells and mega-karyocytes within the solid portions of the mass when compared to the normal adjacent liver parenchyma. This is a commonly reported phenomenon of this neoplasm. Although EMH is not uncommon in the fetal liver, the marked increase within the neoplasm compared to the normal liver tissue stimulated some discussion among attendees. The molecular pathogenesis of EMH within hepatoblastomas is not yet known; however, it is thought that the primitive neoplastic cells may retain the CD34 positive multipotential nature of bone marrow origin cells and can differentiate into erythroid precursors and mega-karyocytes under the influence of hematopoietic cytokines such as IL-6, G-CSF, and GM-CS.15 The presence of EMH within the neoplasm can assist in differentiating hepatocellular carcinoma and macrotrabecular hepatoblastoma, which can have a similar morphology.15
References:
- Ano N, Ozaki K, Nomura K, Narama I. Hepatoblastoma in a cat. Vet Pathol. 2011; 48(5):1020-3.
- De Vries C, Vanhaesebrouck E, Govaere J, Hoogewijs M, Bosseler L, Chiers K, Ducatelle R. Congenital ascites due to hepatoblastoma with extensive peritoneal implantation metastases in a premature equine fetus. J Comp Pathol. 2013 Feb;148(2-3):214-9.
- Cullen JM. Tumors of the liver and gallbladder. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Ames, IA: Wiley Blackwell; 2017:853-855.
- Cullen JM, Stalker MJ. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier Ltd; 2016:347-348.
- Falix FA, Aronson DC, Lamers WH, Hiralall JK, Seppen J. DLK1, a serum marker for hepatoblastoma in young infants. Pediatr Blood Cancer. 2012 Oct;59(4):743-5.
- Helmberger TK, Ros PR, Mergo PJ, Tomczak R, Reiser MF. Pediatric liver neoplasms: a radiologic-pathologic correlation. Eur Radiol. 1999;9(7):1339-47.
- Kim Y, Sills RC, Houle CD. Overview of the molecular biology of hepatocellular neoplasms and hepatoblastomas of the mouse liver. Toxicol Pathol. 2005; 33(1):175-80.
- López-Terrada D, Alaggio R, de Dávila MT, Czauderna P, Hiyama E, Katzenstein H, Leuschner I, Malogolowkin M, Meyers R, Ranganathan S, Tanaka Y, Tomlinson G, Fabrè M, Zimmermann A, Finegold MJ; Children's Oncology Group Liver Tumor Committee. Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol. 2014 Mar;27(3):472-91.
- Loynachan AT, Bolin DC, Hong CB, Poonacha KB. Three equine cases of mixed hepatoblastoma with teratoid features. Vet Pathol. 2007 Mar;44(2):211-4.
- Neu SM. Hepatoblastoma in an equine fetus. J Vet Diagn Invest. 1993 Oct;5(4):634-7.
- Nonoyama T, Fullerton F, Reznik G, Bucci TJ, Ward JM Nonoyama T, Fullerton F, Reznik G, Bucci TJ, Ward JM: Mouse hepatoblastomas: a histologic, ultrastructural, and immunohistochemical study. Vet Pathol. 25: 286296, 1988
- Nonoyama T, Reznik G, Bucci TJ, Fullerton F. Hepatoblastoma with squamous differentiation in a B6C3F1 mouse. Vet Pathol. 1986 Sep;23(5):619-22.
- Shiga A, Shirota K, Shida T, Yamada T, Nomura Y. Hepatoblastoma in a dog. J Vet Med Sci. 1997 Dec;59(12):1167-70.
- Tanaka Y, Inoue T, Horie H. International pediatric liver cancer pathological classification: current trend. Int J Clin Oncol. 2013 Dec;18(6):946-54.
- Thambi R, Lekshmi D, et al. Extramedullary hematopoiesis as a clue to diagnosis of hepatoblastoma on fine needle aspiration cytology: A report of two cases. J Cytol. 2013; 30(3):198-200.
- Watt BC, Cooley AJ, Darien BJ. Congenital hepatoblastoma in a neonatal alpaca cria. Can Vet J. 2001 Nov;42(11):872-4.
- Weng YR, Cui Y, Fang JY. Biological functions of cytokeratin 18 in cancer. Mol Cancer Res. 2012 Apr;10(4):485-93.
- Wright JR Jr, Pinto-Rojas A, Trevenen CL, Yu W. Teratoid features in mixed hepatoblastoma. Vet Pathol. 2010 Sep;47(5):1003-4.