CASE 2: S15_8036 (4084865-00)
Signalment:
2-month-old, female, red Holstein calf, Bos taurus, bovine.
History:
The calf was referred to the ruminant clinic with severe emaciation, failure to thrive and long lasting, intermittent diarrhea resistant to therapy. Routine viral, bacteriological and parasitological examinations (Rotavirus, Coronavirus, Salmonella, E.coli, Cryptosporida, Coccidia) were negative. Blood chemistry revealed that cholesterol and triglyceride levels were severely lower (0.18 and 0.06 mmol/L) when compared to healthy animals (1.79-3.20 and 0.27-0.40 mmol/L) in the same age range6. The animal was euthanized because of poor prognosis and suspicion of a genetic defect as a possible cause for the clinical symptoms.
Gross Pathology:
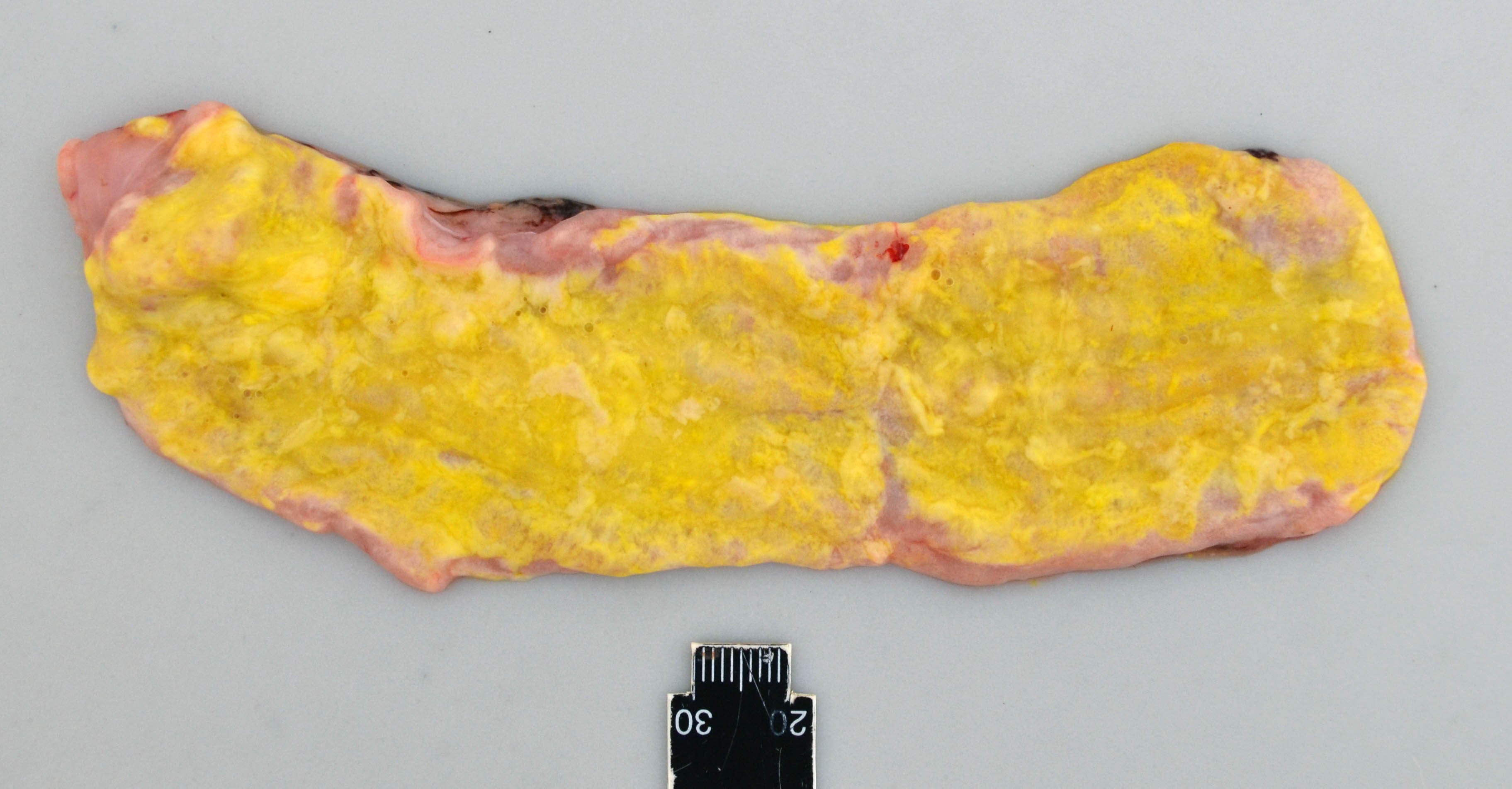
The calf was cachectic and the perianal region was smeared with yellow to green colored feces.
The small and large intestine were diffusely filled with a moderate to large amount of foamy and greasy, liquid content consistent with steatorrhea. The color varied from bright yellow in the proximal parts of the gastro-intestinal tract, to more green color distally. The mucosa, especially of the jejunum, was diffusely edematous and appeared thickened and whitish.
Laboratory results:
Genetic analysis revealed a 1.3 kb insertion within the coding sequence of the apolipoprotein B (APOB) gene.
Microscopic description:
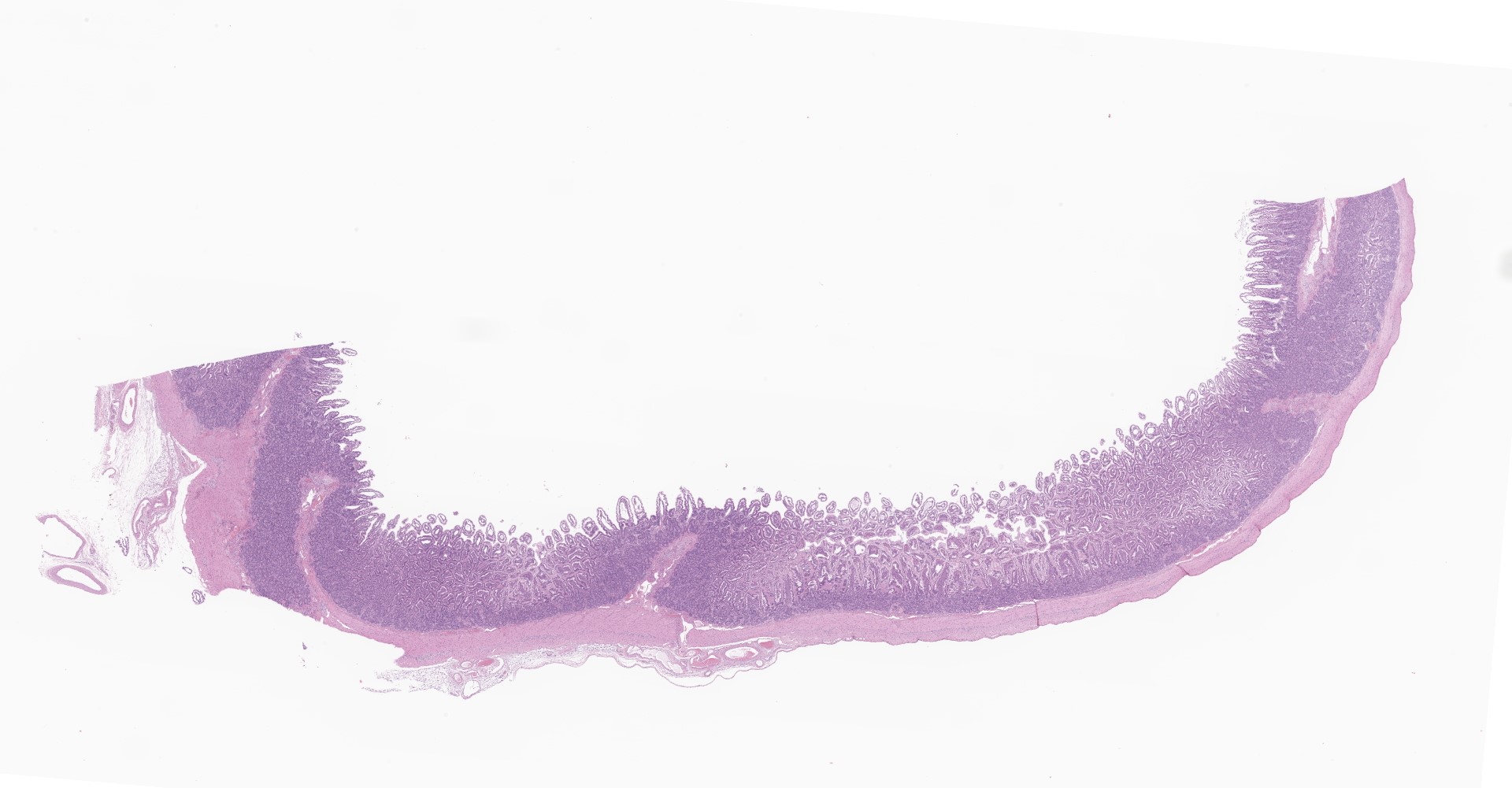
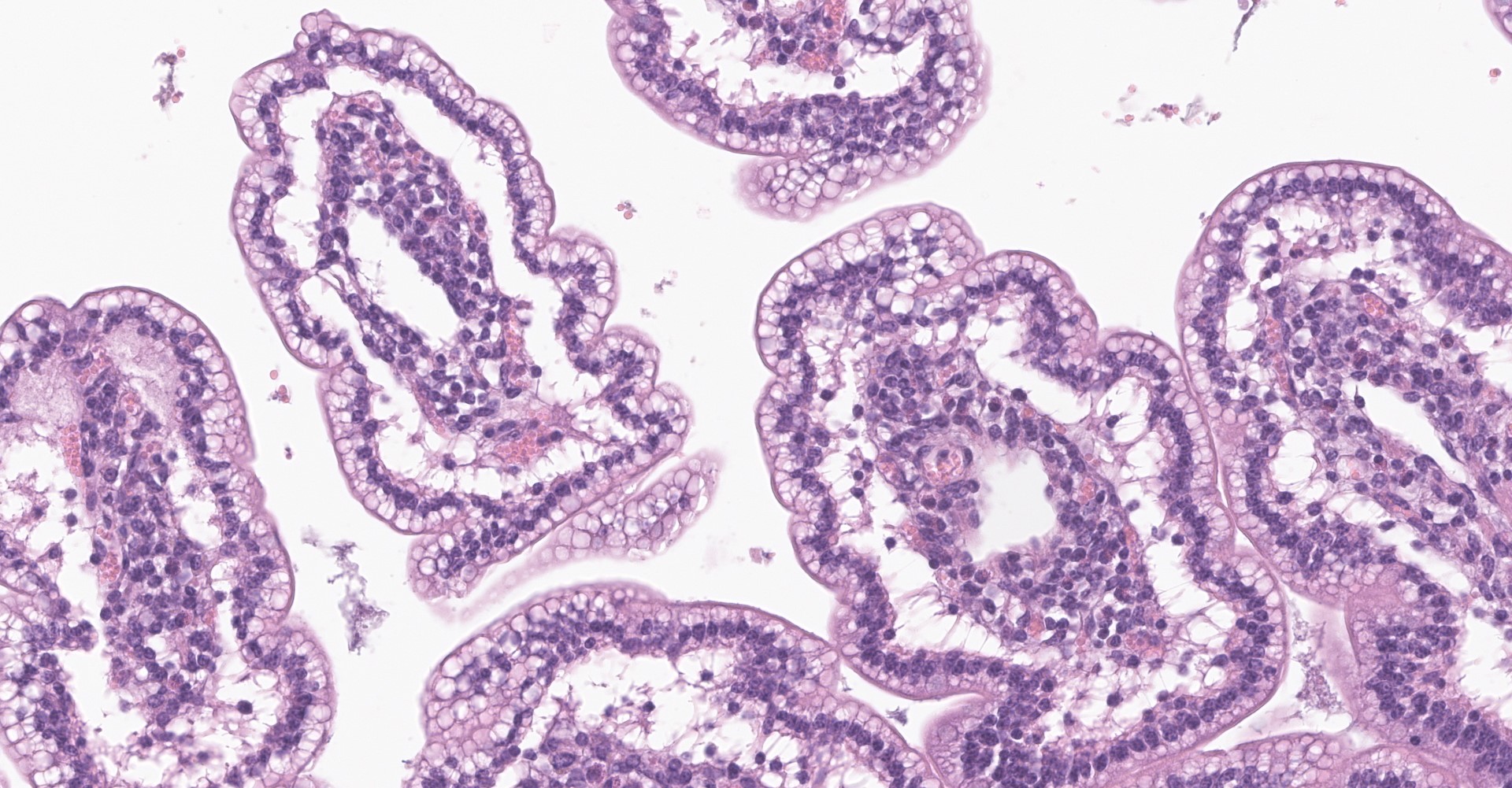
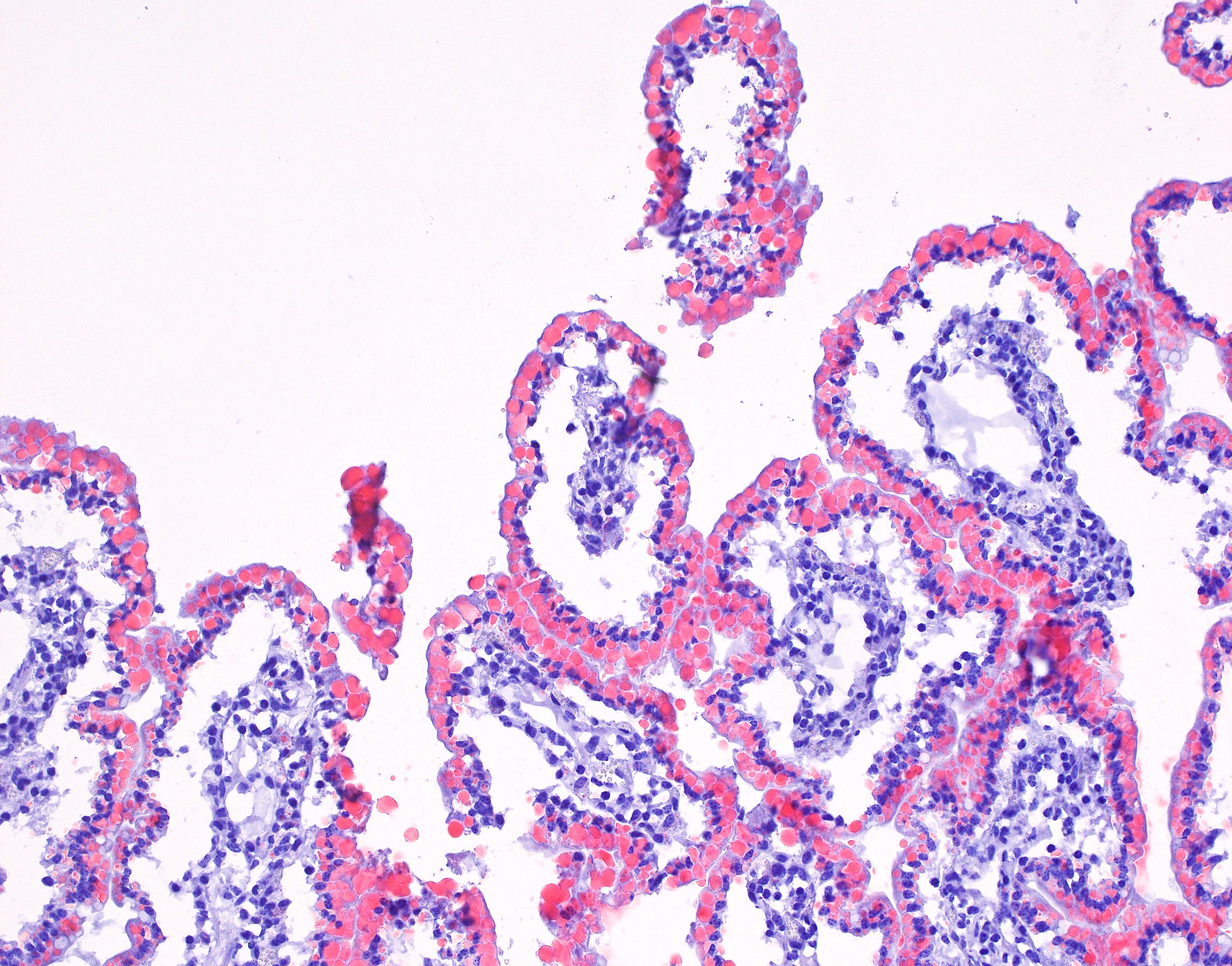
Small intestine (jejunum): Diffusely within the enterocytes covering the tips of the villi, optically empty, round to oval vacuoles varying from approximately 2 to 20 µm in diameter are present (fat vacuoles). Occasionally the vacuoles are located subnuclearly and dislocate the nucleus to the apical cell border. Within the lamina propria, multifocal to coalescing areas of loose, optically empty spaces (edema) are present and the lacteals
are dilated. The amount of lymphocytes within the submucosa is slightly increased.
The optically empty vacuoles stain positive by the Sudan stain for lipids in frozen sections.
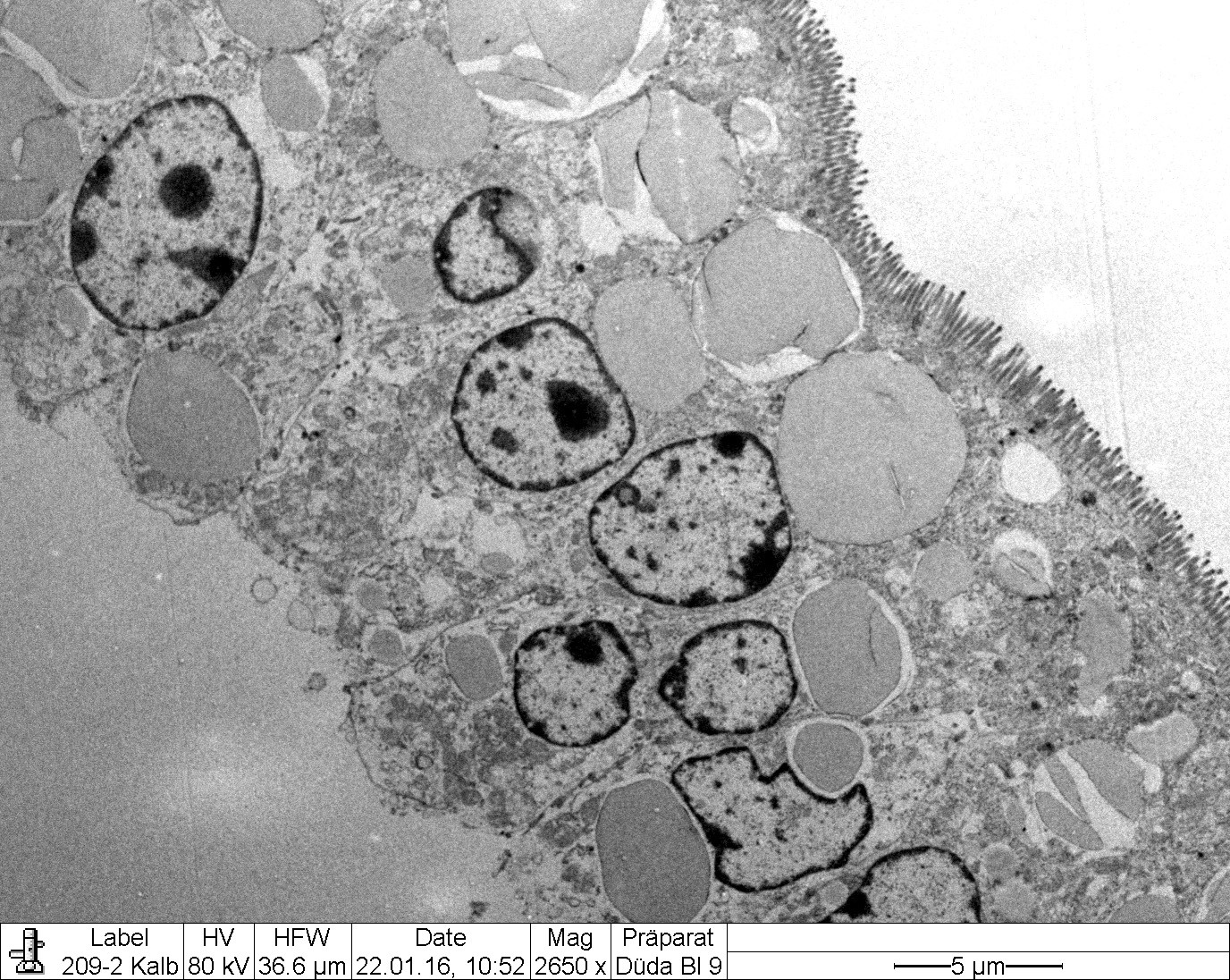
Transmission electron microscopy (Figure 2-6) corroborated the presence of large intracytoplasmic lipid droplets in the small intestinal enterocytes. The droplets filled most of the supranuclear cytoplasm but also occurred in an infranuclear position (Fig. EMCD1).
Contributor's morphologic diagnosis:
Small intestine (jejunum) HE and TEM: Diffuse, severe, intracytoplasmic vacuolization (lipid retention) with moderate, diffuse edema.
Contributor's comment:
The inherited autosomal recessive genetic defect affecting Holstein calves, named cholesterol deficiency (CD), was reported for the first time in the Summer of 2015 in Germany.5 Homozygous calves demonstrate clinical signs of diarrhea unresponsive to treatment and failure to thrive.5 They suffer marked hypocholesterolemia and hypolipidemia, indicating an inherited fat metabolism disorder. These animals usually die within the first six months of life and it
has been assumed that about 80% of homozygous affected calves do not survive more than one year.5 Heterozygous carrier animals do not show any clinical signs but have reduced levels of blood cholesterol and triglycerides.5 Breeding organizations in Switzerland and other countries have reported an increasing occurrence of cases in Holstein cattle. The causal mutation has recently been identified in the apolipoprotein B gene (APOB).7 This case is one out of the series describing the pathological phenotype of CD for the first time.8
Pedigree analysis revealed the Canadian Holstein bull Maughlin Storm as the first carrier for this disorder.5,7 The mutation in this case represents a 1.3 kb insertion of a transposable LTR element (ERV2-1) in the coding sequence of the APOB gene, which leads to truncated transcripts and aberrant splicing.7
The encoded apolipoprotein B (APOB) protein is an essential apolipoprotein of chylomicrons and low-density lipoproteins. The mutation represents a loss of function mutation, similar to the autosomal recessive inherited familial hypobetalipoproteinemia-1 (FHBL1) in humans.7 The APOB gene encodes two proteins via a mRNA editing process: the APOB-48 protein is required for chylomicron production and cellular transport of lipids in the small intestine, and the APOB-100 protein which is expressed in the liver. The APOB-100 protein is a structural component of very low density lipoprotein (VLDL) and its metabolic products, and serves as the ligand for low density lipoprotein (LDL)-receptor mediated endocytosis of lipid particles.3
Truncation mutations in the APOB-48 protein, which is exclusively synthesized in the enterocytes of the small intestine4, were shown to result in defective chylomicron formation in the small intestine.3,9 The histologically visible accumulation of lipid vacuoles within the enterocytes in the CD-affected cattle indicates that the enterocytes of these animals are capable of resorbing fat from the ingesta. However, the hypocholesterolemia and low triglyceride concentrations demonstrate that the transfer of these lipids from the enterocyte into the blood is impaired or even absent. The clinical and pathological findings support this mechanism. In human patients, FHBL1 is additionally characterized by chronic malabsorption of lipid-soluble vitamins, leading to retinal degeneration, neuropathy and coagulopathy.6
The gross findings include steatorrhea (diarrhea) and an edematous mucosa of the small intestine. The animals are usually cachectic. The histological findings are limited to the small intestine and consist of lipid vacuoles within the enterocytes as well as dilated lacteals.8
Taking the pathological findings of steatorrhea, the histological and TEM phenotype of lipid vacuole accumulation within the enterocytes of the tips of the villi in the small intestine and the clinical symptoms of hypocholesterolemia, low triglyceride concentration and the neurological symptoms into account, bovine CD is highly similar to human FHBL.3,9
The main limitations for the diagnosis of CD in cattle are the unspecific clinical symptoms of diarrhea and failure to thrive in young calves. It is important to first exclude common agents causing diarrhea. In case of intermittent diarrhea resistant to treatment in young Holstein cattle, analysis of total cholesterol and triglycerides can lead to a suspicion of CD. Pathological investigations are only diagnostic if samples of the small intestine are immediately fixed in formalin (within minutes after euthanasia). Otherwise, the lipid vacuoles in the enterocytes of the villi tips are lost due to immediate autolytic changes in the small intestine. A clinical or pathological suspicion of CD should be confirmed using a gene test detecting the APOB gene mutation after consideration of pedigree information indicating inbreeding linked to the founder sire Maughlin Storm.8
Contributing Institution:
Institute of Animal Pathology
Vetsuisse Faculty, University of Bern
Laenggassstrasse 122
CH-3012, Bern
Switzerland
http://www.itpa.vetsuisse.unibe.ch/
JPC diagnosis:
1. Small intestine: Villar enterocyte lipidosis, diffuse, marked, with lacteal dilation.
2. Adipose tissue: Atrophy, diffuse, moderate.
JPC comment:
The contributor provides an excellent overview of the homozygous apolipoprotein B gene (APOB) mutation associated with cholesterol deficiency in Holstein calves.
When initially described in 2016, the APOB mutation was only thought to induce clinical signs in homozygous animals, although heterozygotes were noted to have reduced levels of blood cholesterol and triglycerides.5 A subsequent study identified 18 cases of heterozygous Holstein calves with history of poor development, low weight, and intermittent diarrhea with concurrent reduced cholesterol and triglyceride blood concentrations.2 These animals tested negative for rotavirus, coronavirus, bovine viral diarrhea virus, coccida, cryptosoporida, E. coli, and Salmonella spp. and symptomatic treatments did not lead to clinical improvement. Given the reduced cholesterol and triglyceride blood concentrations and lack of other plausible explanation, these cases likely indicate the mutation acts as incomplete dominance with reduced penetrance in heterozygotes.2
An additional study evaluating the lactational and reproductive performance of heterozygous Holstein cows predictably identified markedly lower circulating levels of cholesterol and cholesterol in lipoprotein fractions compared to noncarriers.1 However, lactational and reproductive performance was not impaired compared to noncarriers. The authors therefore found no need to eradicate APOB carriers from production, though risk-matings of carriers should be avoided.1
References:
1. Gross JJ, Schwinn AC, Schmitz-Hsu F, et al. The APOB loss-of-function mutation of Holstein dairy cattle does not cause a deficiency of cholesterol but decreases the capacity for cholesterol transport in circulation. J Dairy Sci. 2019;102(11):10564-10572.
2. Häfliger IM, Hofstetter S, Mock T, et al. APOB-associated cholesterol deficiency in Holstein cattle is not a simple recessive disease. Anim Genet. 2019;50(4):372-375.
3. Hooper AJ, van Bockxmeer FM, Burnett JR. Monogenic hypocholesterolaemic lipid disorders and apolipoprotein B metabolism. Crit Rev Clin Lab Sci 2005;42:515?545.
4. Kane JP, Hardman DA, Paulus HE. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A 1980;77:2465?2469.
5. Kipp S, Segelke D, Schierenbeck S, et al. A new Holstein Haplotype affecting calf survival. Interbull Bull 2015; 49:49-53
6. Lee J, Hegele RA. Abetalipoproteinemia and homozygous hypobetalipoproteinemia: a framework for diagnosis and management. J Inherit Metab Dis 2014;37:333?339.
7. Menzi F, Besuchet-Schmutz N, Fragnier M, et al. A transposable element insertion in APOB causes cholesterol deficiency in Holstein cattle. Anim Genet 2016; 47:253-257.
8. Mock T., Mehinagic K., Menzi F., et al. Clinicopathological Phenotype of Autosomal Recessive Cholesterol Deficiency in Holstein Cattle. J Vet Intern Med 2016 Submitted January 27, 2016; Revised March 14, 2016; Accepted.
9. Welty FK. Hypobetalipoproteinemia and abetalipoproteinemia. Curr Opin Lipidol 2014;25:161?168.