Signalment:
Gross Description:
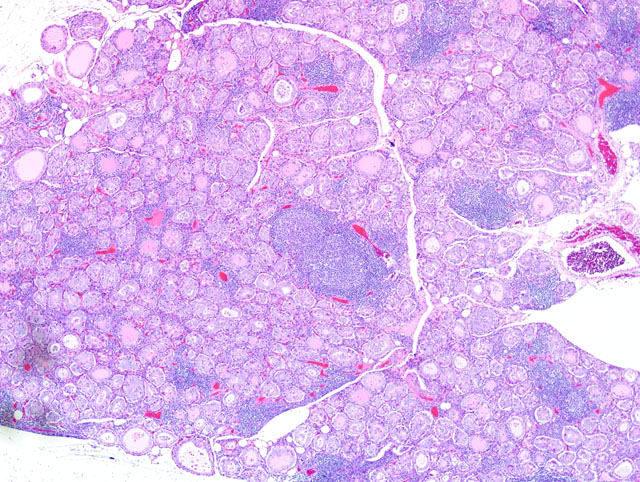
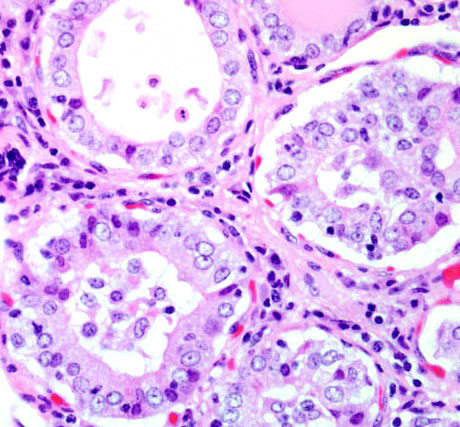
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
| Test | Result | Humans Reference Range6 | ||
| Total Thyroxine (TT4) | 14 | 60-140 nmol/L | ||
| Total Triiodothyronine (TT3) | 2.3 | 1.1-2.7 nmol/L | ||
| Free Thyroxine (FT4) | 7 | 10-25 pmol/L | ||
| Free Triidothyronine (FT3)) | 6.8 | 3-8 pmol/L | ||
| T4 Autoantibody* | 15% | N/A | ||
| T3 Autoantibody* | 3% | N/A |
* Autoantibodies to T3 and T4 are presented as the percentage of hormone binding relative to each antibody negative control
Condition:
Contributor Comment:
Two types of autoimmune thyroiditis are described in humans, Graves disease and Hashimotos thyroiditis, the latter being more frequent.(3,4,6,8) In Graves disease, the inflammation is mild, while the thyroid glands are enlarged due to proliferation of thyrocytes, resulting in follicular hyperplasia and hypertrophy. Graves disease accounts for 50-80% of hyperthyroidism cases in humans, and results from circulating IgG antibodies that bind to and activate the G-protein-coupled thyrotropin receptor. Clinical pathological findings include decreased serum thyrotropin, elevated serum triiodothyronine (T3) and thyroxine (T4), with an increased fraction of T3 relative to T4.(1,3,6)
In Hashimotos thyroiditis, the lymphocytic infiltration is prominent, often with germinal center formation, causing enlargement of the thyroid glands and destruction of the thyroid follicles, leading to hypothyroidism. The pathogenesis of Hashimotos thyroiditis involves a delayed hypersensitivity reaction to thyroid epitopes. Sensitization of autoreactive CD4+ T-helper cells to these initiates the immunologic events leading to thyrocyte death. The effector mechanisms include destruction of thyrocytes by CD8+ cytotoxic T cells by exocytosis of perforin/granzyme granules or engagement of the death receptor, overproduction of inflammatory cytokines by CD4+ T cells, and the binding of antithyroid antibodies (anti-TSH receptor, antithyroglobulin, and antithyroid peroxidase antibodies) followed by antibody-dependent cell-mediated cytotoxicity. Hypothyroidism usually develops gradually, although in some cases it may be preceded by transient thyrotoxicosis due to the disruption of thyroid follicles, with secondary release of thyroid hormones (hashitoxicosis).(2,3,6,8)
As there is relatively little literature on African green monkeys, normal reference ranges for humans (7) were used to confirm the presence of autoimmune thyroiditis in this case. Serum-binding assays to measure thyroxine (T4) and triiodothyronine (T3) autoantibodies revealed15% and 3% more binding of the immunoglobulin to the T4 and T3, respectively, compare to each negative control. Low TT4 and FT4 support a diagnosis of hypothyroidism, which, combined with the histological appearance, resembles Hashimotos thyroiditis in humans. Values of thyroid stimulating hormone (TSH), autoantibodies against thyroglobulin, thyroperoxidase, or TSH receptor are not available due to the limitation of serum available for testing.Â
JPC Diagnosis:
Conference Comment:
Gross lesions in dogs with lymphocytic thyroiditis vary and include normal sized, enlarged or hypoplastic thyroid glands that may be discolored tan to off white. The classic histologic appearance is of multifocal to diffuse infiltrates of lymphocytes and plasma cells that separate thyroid follicles and may form lymphoid nodules. Follicles are often shrunken and lined by columnar epithelial cells, which may cause nests of C cells to appear more prominent between follicles.(2)
Migration of lymphocytes and plasma cells between follicles causes follicular cells to lose their attachment to the basement membrane, and this leads to sloughing of cells into the lumen of the follicle. Lymphoid cells also migrate into the lumen, and this combination of changes leads to eventual death of the follicle. While the damage to follicles is ongoing, adjacent follicles undergo hypertrophy in an attempt to keep up with demand and in response to increased TSH secretion. Eventually the parenchyma of the thyroid gland is replaced by fibrous connective tissue. At this stage, only scant residual inflammatory cells and small, widely dispersed end-stage follicles with a small amount of vacuolated colloid remain.(2)
References:
2. Capen CC: Endocrine Glands. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol.3, p386. Elsevier Saunders, Philadelphia, USA, 2007
3. Caturegli P, Kimura H, Rocchi R, Rose NR: Autoimmune thyroid diseases. Curr Opin Rheumatol 19(1):44-8, 2007
4.Guzman RE, Radi ZA: Chronic lymphocytic thyroiditis in a cynomolgus monkey (Macaca fascicularis). Tox Pathol 35:296-299, 2007
5. La Perle KMD, Capen CC: Endocrine system. In: Pathologic Basis of Veterinary Disease, ed McGavinMD, Zachary JF, 4th ed., pp.722. Mosby Elsevier, St. Louis, MO 2007
6. Maitra A, Abbas AK: The Endocrine System. In: Robbins and Cotran Pathologic Basis of Disease, ed. Kumar V, Abbas AK, Fausto N, 7th ed., pp1169-73. Elsevier Saunders, Philadelphia, USA, 2005
7. Stockigt J: Assessment of thyroid function: Towards an integrated laboratory clinical approach. Clin Biochem Rev 24:110-23, 2003
8. Wang SH, Baker JR: The role of apoptosis in thyroid autoimmunity. Thyroid 17(10):975-9, 2007