WSC 22-23
Conference 2
CASE I: P21-0286 (JPC 4166941)
Signalment:
A three-year-old female pot-bellied pig (Sus scrofa domesticus)
History:
Two-week history of intermittent pelvic limb lameness and abnormal mentation. Brought to the Veterinary Teaching Hospital unable to ambulate in rear. Neuro exam w/CT scan indicated multiple cerebellar masses. Owner elected euthanasia due to deteriorating neurological condition of patient and poor prognosis.
Gross Pathology:
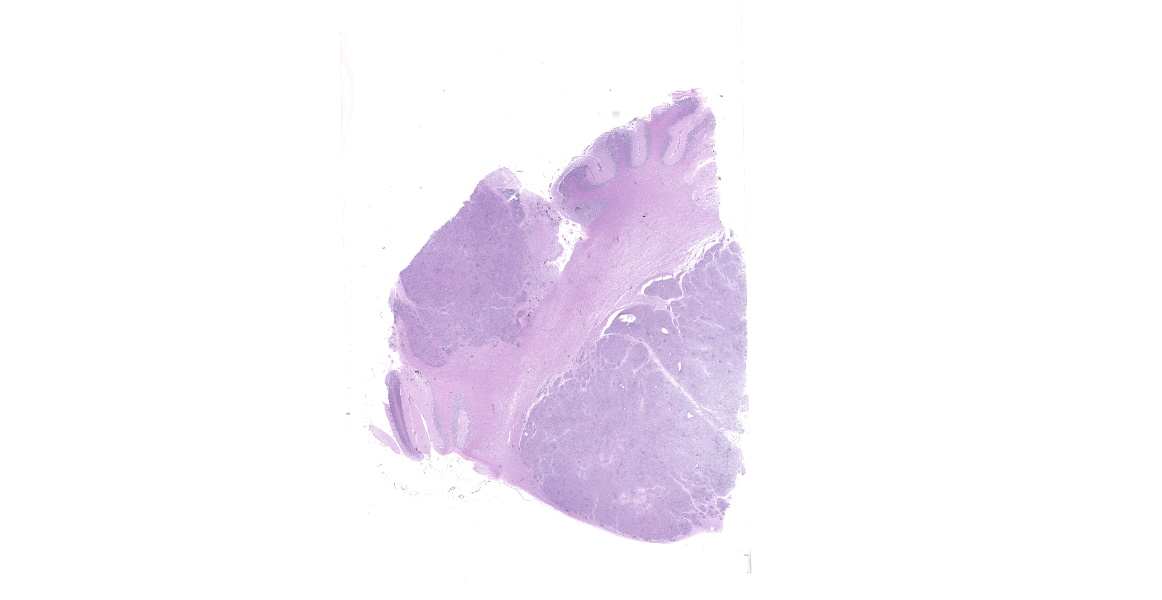
Brain: The gyri and sulci are flattened throughout the cerebrum. When removed the cerebrum leaks a large amount of clear cerebrospinal fluid and the cerebrum flattens. The cerebellum is cone-shaped and pushed out the foramen magnum. The vermis is flattened and has loss of detail. After fixation the brain is sectioned. The lateral ventricles are severely dilated and the gray and white matter of the cerebrum compressed. There are several pale tan nodules that distort the cerebellum.
Laboratory Results:
Scrolls were cut from formalin-fixed, paraffin embedded blocks of cerebellum. DNA was extracted using the Qiagen QIAmp DNA FFPE Tissue kit according to the manufacturer?s directions. Extracted DNA was amplified using forward and reverse primers for the 16S-23S rRNA gene spacer as published.1 The amplicon was submitted for Sanger sequencing with the product submitted for a BLAST search. The sequence had highest identity (99.63%) with Mycobacterium avium subsp. hominissuis.
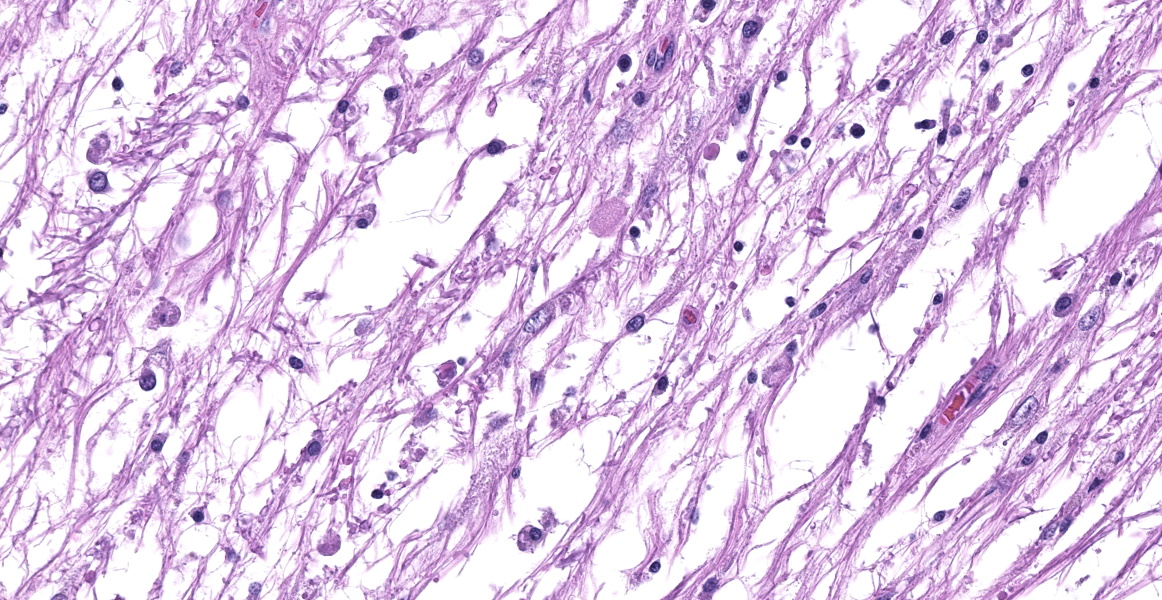
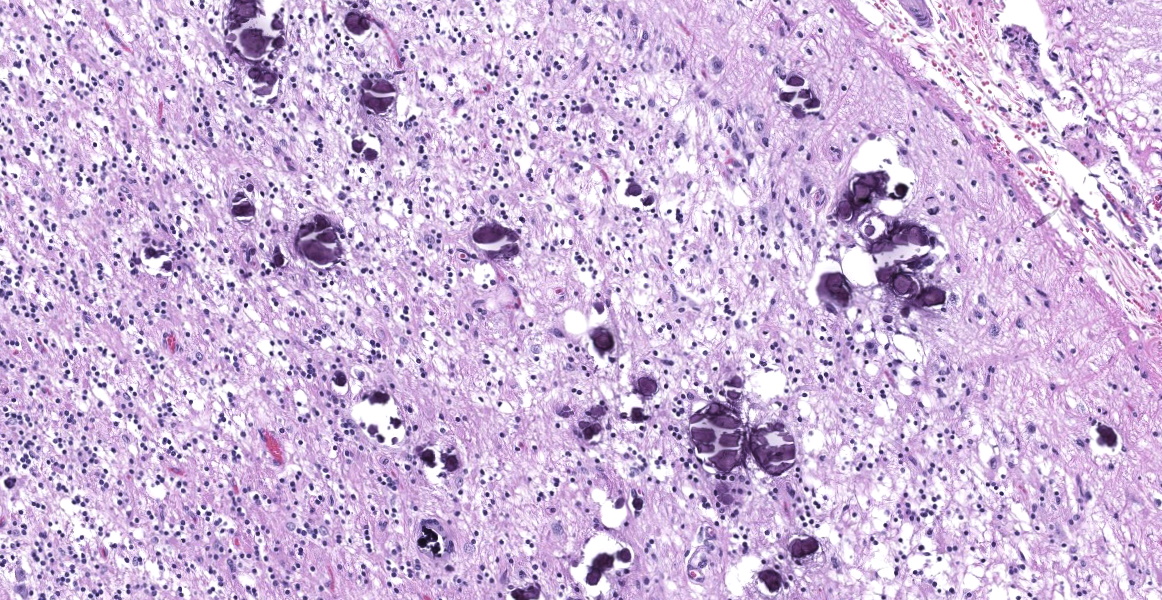
Microscopic Description:
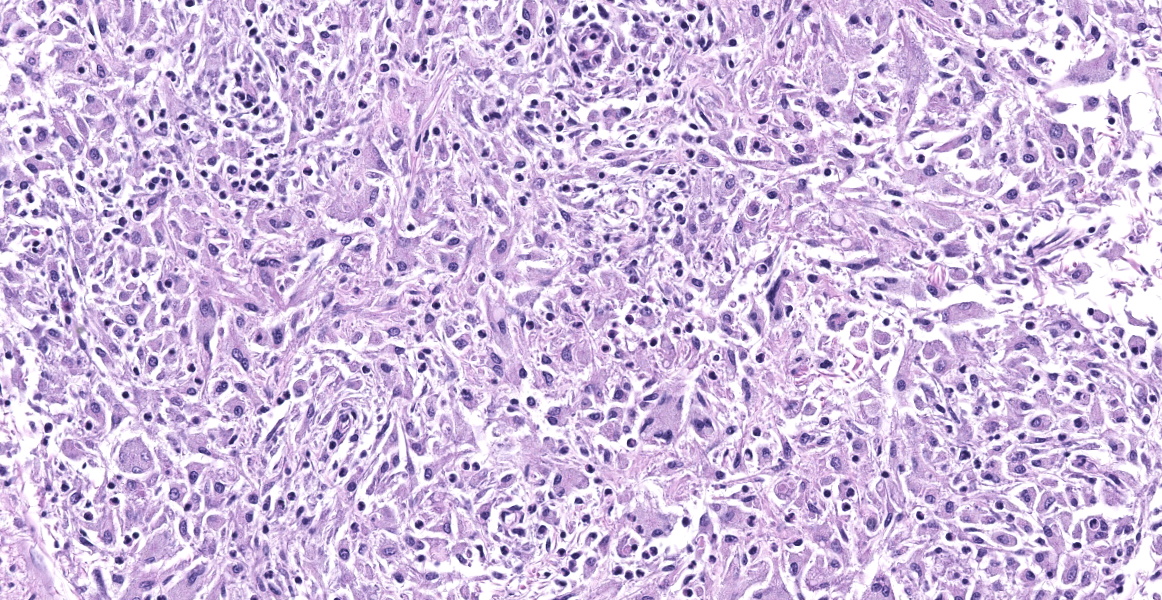
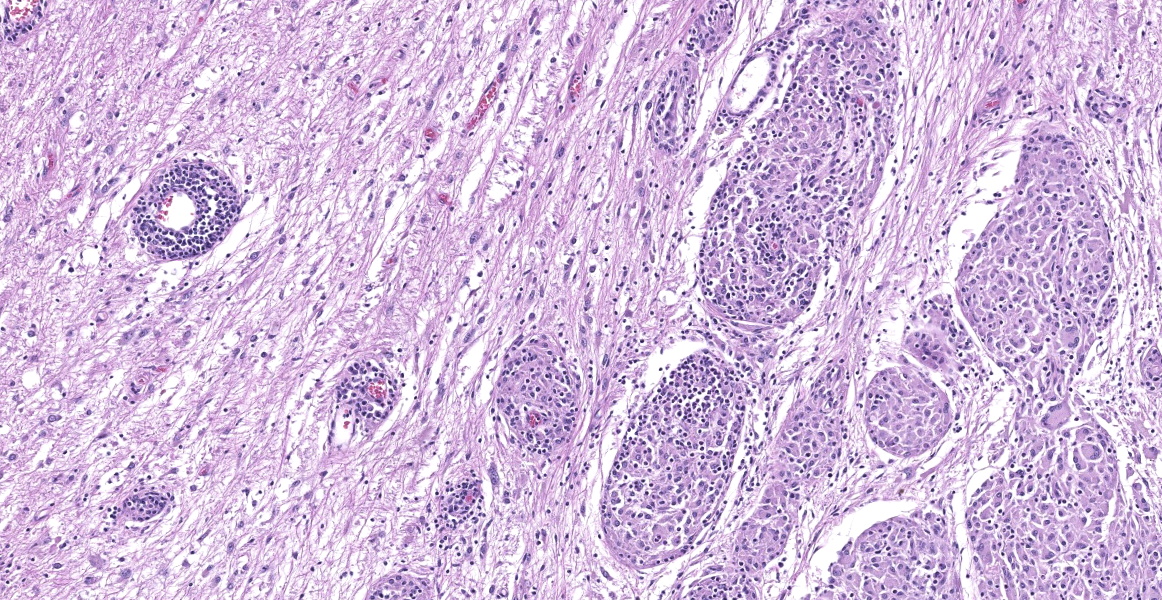
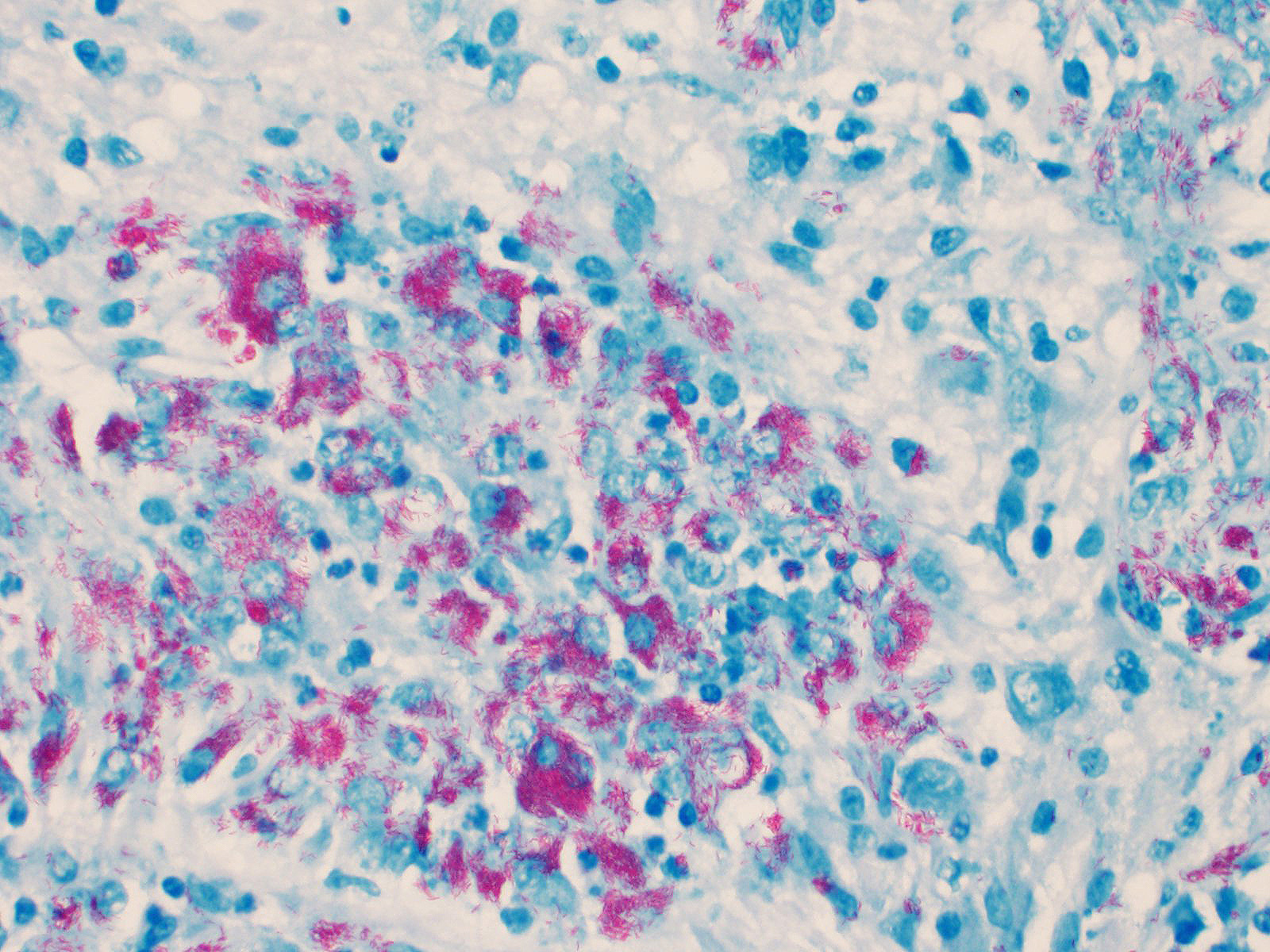
Cerebellum: The cerebellar gray and white matter are effaced by large, unencapsulated, compressive nodules composed of large numbers of epithelioid macrophages and multinucleate giant cells with peripheral nuclei. There are smaller numbers of neutrophils, lymphocytes and eosinophils scattered within the nodules. Some of the larger cells have slight heterogeneity of the eosinophilic cytoplasm. Staining with acid fast stain reveals large numbers of bacilli with in the cytoplasm of macrophages and multinucleate giant cells. Large clusters of lymphocytes and plasma cells surround medium-caliber blood vessels.
Contributor's Morphologic Diagnoses:
Brain:
- Granulomas, multifocal, chronic, severe, cerebellum with intralesional acid fast bacteria
- Hydrocephalus, diffuse, severe with cerebellar coning
Contributor's Comment:
At the time of necropsy, cerebellar neoplasia was the primary differential based on MRI and gross findings of space occupying masses that compressed cerebral spinal fluid flow and led to secondary hydrocephalus. On histologic review, granulomatous inflammation was identified and slight granularity of the cytoplasm of epithelioid macrophages and multinucleate giant cells suggested intracellular organisms. Small bacilli consistent with mycobacteria were confirmed with acid-fast staining. Because there was no fresh tissue for culture, the organisms were identified using PCR and Sanger sequencing.7 Mycobacterium avium subsp. hominissuis was identified. Deep sequencing was attempted, but host nucleic acid predominated and no reads corresponding to mycobacteria were identified.
Mycobacteria are acid-fast, non-motile, non-spore forming, intracellular, aerobic bacilli. Mycobacterium avium subsp. hominisuis is a member of the M. avium complex (MAC) as compared to other mycobacteria in the M. tuberculosis complex with members such as M. bovis, M. tuberculosis, and M. microti. MAC is primarily composed of M. avium subspecies and M. intracellulare and are commonly found in fresh and salt water as well as soil.9 Infections are typically associated with immunocompromise in the mammalian host and are typically not contagious. M. avium subspecies hominissuis is most frequently isolated from humans and pigs as suggested by the nomenclature.
While reports of mycobacterial lymphadenitis are common and worldwide,9 to our knowledge, the only report of mycobacteriosis in pot-bellied pigs is a single case report of M. kansasii, another member of MAC.8 That case report, as well as reports in other porcine species, typically present as disseminated disease with colonization and inflammation most prominent in lung and lymph nodes.3 This case is unusual in that the granulomas and organisms were only identified in the cerebellum without lymphadenitis or lesions in thoracic or abdominal tissues. There was no indication of underlying disease or immunocompromise.
Contributing Institution:
Virginia Maryland College of Veterinary Medicine.
205 Duck Pond Dr
Blacksburg, VA 24061
https://vetmed.vt.edu/departments/biomedical-sciences-and-pathobiology.html
JPC Diagnosis:
Cerebellum: Meningoencephalitis, granulomatous, multifocal to coalescing, severe, with innumerable intrahistiocytic bacilli.
JPC Comment:
Conference participants discussed differentials for granulomatous disease in this case, including mycobacterial and fungal infections. During the pre-conference lecture, the moderator, LTC Erica Barkei, described a case of Mycobacterium tuberculosis she diagnosed in a rhesus macaque; the paucibacillary lesions in that case provided a striking contrast to the abundant acid fast bacteria in the multibacillary granulomas seen in this case. The moderator explained that the mycolic acid within cell walls impart the acid-fast character of mycobacteria, though they can also be weakly Gram positive, as demonstrated with B&H staining in this case.
Bacteria of the family Mycobacteriaceae may be classified according to the spectrum of disease they cause or their ability to grow in culture. The tuberculous group is composed of closely related zoonotic obligate pathogens M. tuberculosis, M. bovis, and M. microti.5,6 Tuberculous mycobacteria elicit a Th1 response, where interferon gamma stimulates cell mediated immunity and the increased bacteriocidal activity of macrophages results in few bacteria within lesions. IFN-gamma also leads to secondary tissue damage, with granuloma formation or potentially caseous necrosis.2
Another group of obligate pathogens is the leprosy group, which includes M. lepraemurium and M. visibilis.5 In humans, leprosy may incite a tuberculoid (Th1) response, as previously described, or a lepromatous response, in which there is a weak Th1 response and variable Th2 response. In these cases, there is weak cell-mediated immunity, abundant bacteria within lesions, and potential production of non-protective antibodies which lead to antigen-antibody complex deposition.2
The nontuberculous group includes, among others, the M. avium complex (MAC) with subspecies avium, sylvaticum, paratuberculosis, and hominissuis.1 As the contributor mentioned, MAC bacteria are environmental opportunistic pathogens and infection is generally localized to the skin unless there is concurrent immunocompromise.5 MAC bacteria grow slowly in culture but lesions in animals contain generally contain abundant bacteria.5 Other nontuberculous mycobacteria which grow rapidly in culture can cause atypical mycobacteriosis, particularly in cats. The lesions similarly depend on the immune status of the animal and vary from chronic panniculitis, granulomatous pneumonia, to systemic disease in immunosuppressed animals.5 Few bacteria are found within the lesions of atypical mycobacteriosis.5
In general, poultry and swine are considered more susceptible to MAC, whereas dogs, cats, and domestic rabbits are more resistant.1,3 Literature on primary M. avium hominissuis (MAH) in domestic animals is sparse; however a couple of recent reports have described MAH infection in a cat and a rabbit without evidence of underlying immunosuppression. The feline case involved a young cat with chronic neurologic signs, pyogranulomatous meningoencephatlitis, and generalized granulomatous lymphadenitis with abundant acid-fast bacteria found in multiple organ systems.4 The rabbit had a focal fibrinonecrotic ulceration within the cecum characterized by granulomatous inflammation, multinucleated giant cells, and intra- and extra-cellular acid-fast bacteria.1 Granulomatous inflammation was also observed in the spleen, liver, lungs, and cecal lymph node, and only the enlarged cecal lymph node contained bacteria, which were scant. In both the cases, M. avium subsp. hominissuis was identified.1,4
References:
- Bertram CA, Barth SA, Clockner B, et a. Intestinal Mycobacterium avium Infection in Pet Dwarf Rabbits (Oryctolagus cuniculus). Comp Path. 2020; 180:73-78.
- Frank KM, McAdam AJ. Infectious Diseases. In: Kumar V, Abbas AK, Aster JC, eds. Robins & Cotran Pathologic Bases of Disease. 10th Philadelphia, PA: Elsevier, Inc; 2021: 339-404.
- Greene CE, Gunn-Moore DA. Mycobacterial Infections: Infections Caused by Slow-growing Mycobacteria. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 4th St. Louis, MO: Elsevier Saunders; 2012: 495-513.
- Madarame H, Saito M, Ogiara K, et al. Mycobacterium avium hominissuis meningoencephalitis in a cat. Vet Micro. 2017; 204:43-45.
- Mauldin EA, Peters-Kennedy A. Integumentary System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer?s Pathology of Domestic Animals. Vol 1. 6th Philadelphia, PA: Elsevier Saunders; 2016: 639-641.
- Pekkarinen H, Airas N, Savolainen LE, et a. Non-tuberculous Mycobacteria can Cause Disseminated Mycobacteriosis in Cats. J Comp Path. 2018; 160:1-9.
- Roth A, Reischl U, Streubel A, et al. Novel diagnostic algorithm for identification of mycobacteria using genus-specific amplification of the 16S-23S rRNA gene spacer and restriction endonucleases. J Clin Microbiol. 2000:38: 1094-1104.
- Schafbuch R, Tinkler S, Lim CK, et al. Disseminated mycobacteriosis caused by Mycobacterium kansasii in a pot-bellied pig. J Vet Diagn Invest. 2018:30: 646-650.
- Thorel MF, Huchzermeyer HF, Michel AL. Mycobacterium avium and Mycobacterium intracellulare infection in mammals. Rev Sci Tech. 2001:20: 204-218.