Signalment:
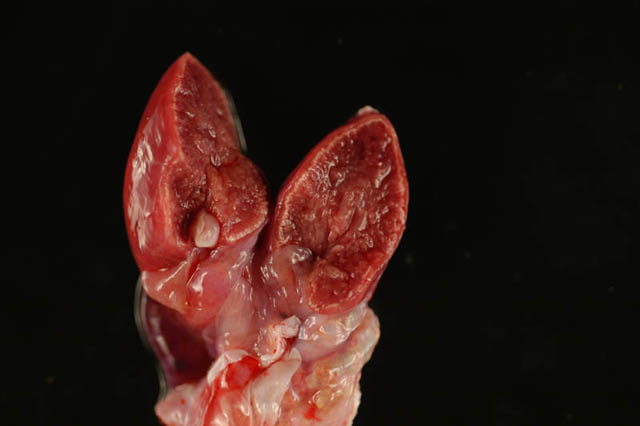
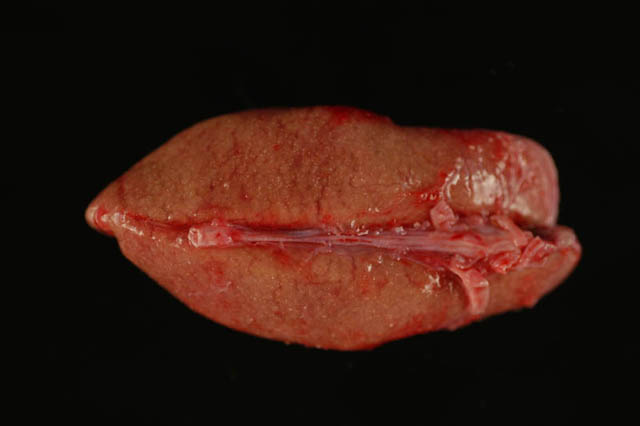
Gross Description:
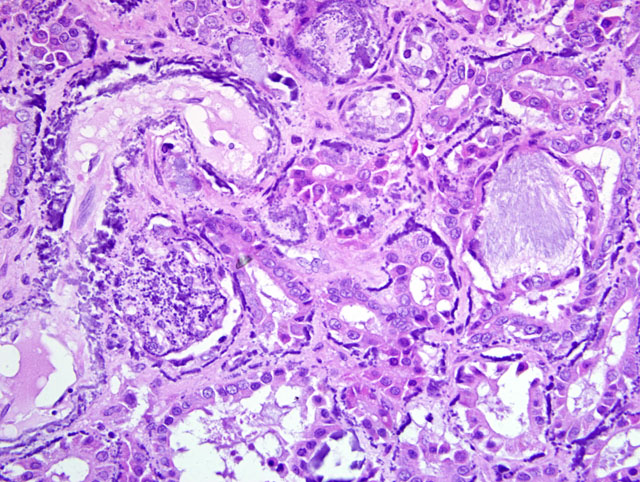
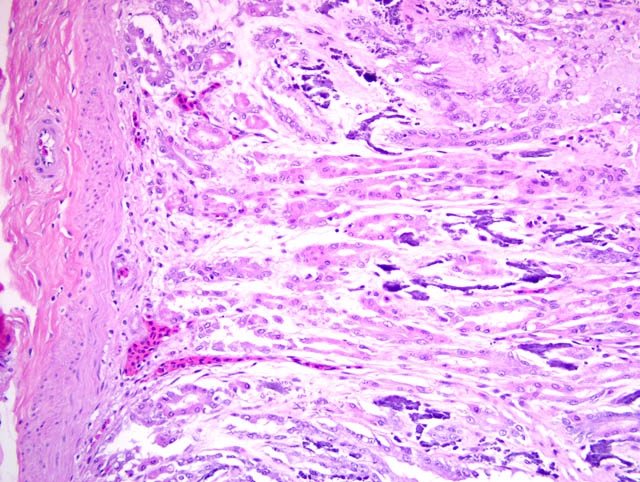
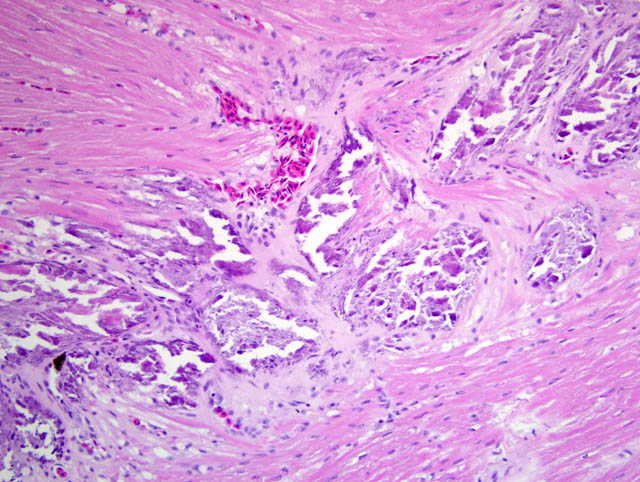
Histopathologic Description:
Multifocally and extensively, there is mineralization of cardiac myofibers. The mineralization is evident as a linear band in the outer myocardium and also as scattered myofibers throughout the myocardium. There is extensive mineralization of the vessels in the myocardium and epicardium. There are moderate amounts of fibrous connective tissue associated with the mineralized myofibers in the linear band region. Surrounding myofibers are frequently markedly vacuolated and occasionally necrotic and infiltrated with moderate numbers of macrophages.
Multifocally, there is often extensive mineralization of the gastric mucosa. The mineralization affects all levels of the mucosa including the luminal and crypt epithelium and the connective tissue of the lamina propria. In more severely mineralized foci, there is often fibrosis in the lamina propria and loss of glands. There is multifocal moderate mineralization of vessels in the submucosa and lamina propria. There is mild mineralization of the outer aspects of the tunica mucosa and the serosa.
Morphologic Diagnosis:
1. Metastatic mineralization, multifocal, chronic, moderate-severe, with:
a. Glomerular, tubular, and vascular mineralization, chronic, multifocal, severe, kidneys.
b. Myocardial and vascular mineralization, chronic, multifocal, marked, with mild myofiber degeneration and necrosis and mild myocardial fibrosis, heart.
c. Gastric mucosal and vascular mineralization, chronic, multifocal, moderate, with fibrosis and loss of glands.
d. Mineralization and fibrosis, chronic, multifocal, severe, aorta (not included in slide set).
2. Renal tubular urate tophus deposition, acute, multifocal, minimal to mild, with tubular necrosis.
Lab Results:
Condition:
Contributor Comment:
Causes of metastatic soft tissue mineralization include hypervitaminosis D (due to excess supplementation or ingestion of vitamin D-containing rodenticides or plants [Cestrum spp. and Solanum spp.]) and elevated calcium and/or phosphorus (nutritional or renal secondary hyperparathyroidism, primary hyperparathyroidism, hypercalcemia of malignancy).(4) Specifically, animals are considered at risk for soft tissue mineralization when the calcium-phosphorus product is greater than 60-70.(4)
Green iguanas require UV-B light to convert provitamin D3 to active vitamin D3, and oral supplementation of vitamin D3 (instead of a UV-B source) has been determined to be ineffectual.(7) A study on iguanas at the National Zoological Park presenting for necropsy revealed widespread mineralization of soft tissues, especially of vessels and basement membranes, cardiac and skeletal muscle degeneration and necrosis with mineralization, and occasional mild fibrous osteodystrophy. In these animals, circulating levels of vitamin D3 were 7-36 ng/ml (normal is >400ng/ml).(7,8)
Possible causes for paradoxical soft tissue mineralization in these animals which were proposed were chronically low vitamin D levels leading to hypocalcemia, resulting in an exaggerated PTH response and excessive calcium mobilization from bone with resultant mineral deposition in soft tissues; or metabolic derangements altering the calcium-phosphorus ratio, which could result in an elevated calcium-phosphorus product which could predispose to mineralization.(7,8)
In this case, [Ca] = 9.8 mg/dL (normal 10.9-14.4), [P] = 30.4 mg/dL (normal 2.8-7.8), and [Ca] x [P] = 297.92. Therefore, in this case, we may have an explanation for mineralization based on elevated [Ca] x [P] product. Vitamin D3 levels were not determined. Given the sub-optimal husbandry reported by the clinician in this case, calcium and phosphorus abnormalities could have been nutritional and/or related to lack of adequate vitamin D3. Renal dysfunction was likely secondary to mineralization. While a few tophi were identified in renal tubules, these were acute lesions without associated inflammatory response, and likely developed terminally, possibly secondary to dehydration.
This animal did not have any evidence of fibrous osteodystrophy.
JPC Diagnosis:
1. Kidney: Glomerular, vascular, and tubular basement membrane mineralization, diffuse, with tubular epithelial necrosis, interstitial fibrosis and few gout tophi.
2. Stomach: Mucosal and vascular mineralization, multifocal, with epithelial degeneration and necrosis and interstitial fibrosis.
3. Heart, ventricle: Myocardial and vascular degeneration, necrosis, and mineralization, multifocally extensive, moderate, with fibrosis.
Conference Comment:
This case is an excellent example of metastatic mineralization of soft tissues, and the contributor provides a succinct review of the pathophysiology of the condition, including hypovitaminosis D in iguanas. Conference participants briefly reviewed the following causes of metastatic mineralization in animals:(1,3,5)
1. Kidney failure → phosphate retention → renal secondary hyperparathyroidism
2. Hypervitaminosis D (e.g. ingestion of calcinogenic plants [Solanum malacoxylon, Cestrum diurnum, Trisetum flavescens] or cholecalciferol-containing rodenticides) → secondary hyperparathyroidism
3. Hyperparathyroidism (primary or secondary) or pseudohyperparathyroidism (due to PTH-related protein production associated with canine lymphoma or adenocarcinoma of the anal sac)
4. Granulomatous disease (e.g. canine blastomycosis, bovine paratuberculosis)
5. Osteolytic bone lesions (e.g. primary or metastatic neoplasia)
In general, tissues predisposed to metastatic mineralization include the gastric mucosa, renal tubules, alveolar septa, and subpleural intercostal connective tissue.(5,6) In cattle with Johnes disease, intimal mineralization may occur in the thoracic aorta.(3) In dogs with blastomycosis, mineralization is thought to be caused by 1,25-dihydroxycholecalciferal production by activated macrophages.(1)
References:
2. Jacobsen ER: Overview of reptile biology, anatomy, and histology. In: Infectious Diseases and Pathology of Reptiles, ed. Jacobsen ER, pp. 13-19, CRC Press, Boca Raton, FL, 2007
3. Maxie MG, Robinson WF: Cardiovascular system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 3, p. 61. Elsevier Saunders, Philadelphia, PA, 2007
4. Morrow CK, Volmer PA: Hypercalcemia, hyperphosphatemia and soft tissue mineralization. Comp Cont Ed Pract Vet 24:380-387, 2002
5. Myers RK, McGavin MD: Cellular and tissue responses to injury. In: Pathologic Basis of Veterinary Disease, ed. McGavin MD, Zachary JF, 4th ed., pp. 48-49. Mosby Elsevier, St. Louis, MO, 2007
6. Newman SJ, Confer AW, Panciera RJ: Urinary system. In: Pathologic Basis of Veterinary Disease, ed. McGavin MD, Zachary JF, 4th ed., pp. 641-644. Mosby Elsevier, St. Louis, MO, 2007
7. Richman LK, Montali RJ, Allen ME, Oftedal, OT: Paradoxical pathologic changes in vitamin D deficient green iguanas (Iguana iguana). In:Proceedings of the American Association of Zoo Veterinarians, pp. 203-204. East Lansing, MI, 1995
8. Richman L, Montali R, Allen M, Oftedal, O: Widespread metastatic soft tissue mineralization in vitamin D deficient green iguanas (Iguana iguana). In: Proceedings of the Conference of the American College of Veterinary Pathology, p. 57. Atlanta, GA, 1995