WSC 2023-2024, Conference 16, Case 1
Signalment:
Three-year-old male intact Lop rabbit (Oryctolagus cuniculus)
History:
The rabbit presented with soft stools and decreased activity. No abnormalities were found on physical examination, except for the presence of loose stools in the perianal area. Treatment with probiotics was initiated. Over the subsequent two days, the rabbit stopped producing fecal pellets and eating. It presented on the third day with no menace response, no gut sounds, and the inability to use the hind limbs. On radiographs, kidneys appeared enlarged. The rabbit died during blood sampling. CPR was attempted but was unsuccessful.
Gross Pathology:
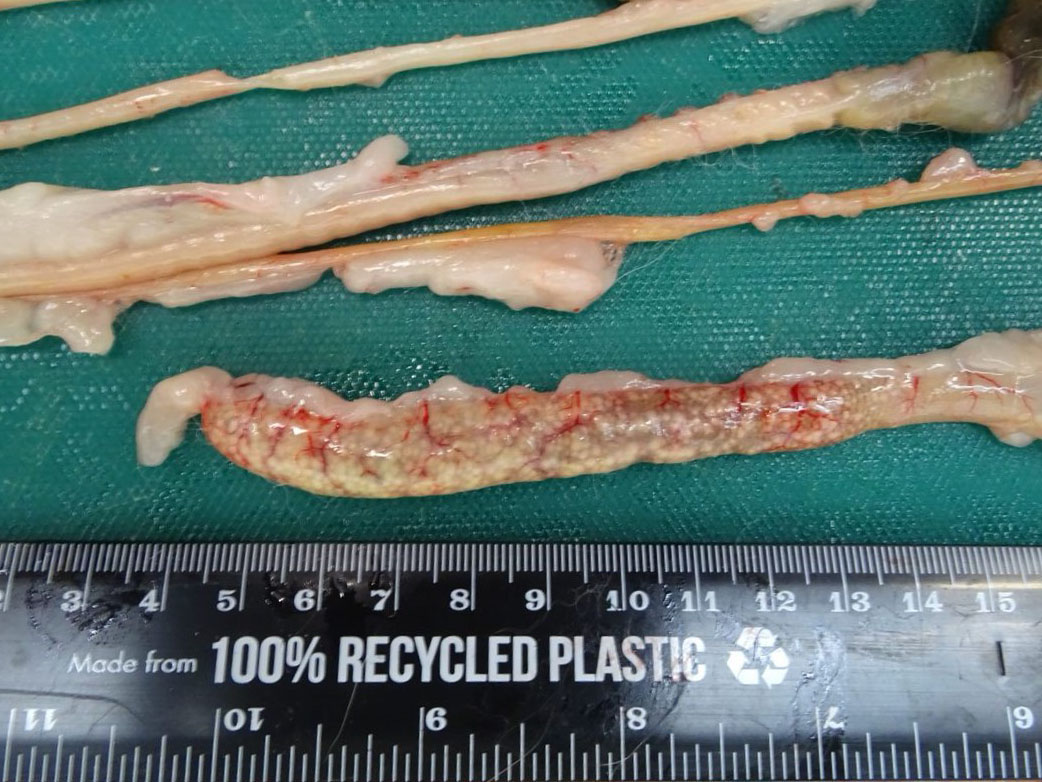
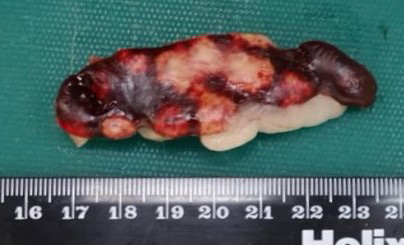
Across all hepatic lobes there were multiple, well demarcated, white foci ranging from <1 to 1 mm in diameter. Affecting approximately 50-60% of the spleen, there were large multifocal to coalescing, circular, poorly delineated, well demarcated firm, cream to white nodules, which extended into the parenchyma and measured up to 9 mm in diameter, and bulged up to 3 mm from the splenic surface. The apical 10 cm of the cecal mucosa and serosa also showed numerous, multifocal to coalescing, white foci, measuring 2-3 mm in diameter, and the cecal wall was moderately thickened.
Laboratory Results:
Microbiological culture of spleen: Moderate growth of Yersinia pseudotuberculosis.
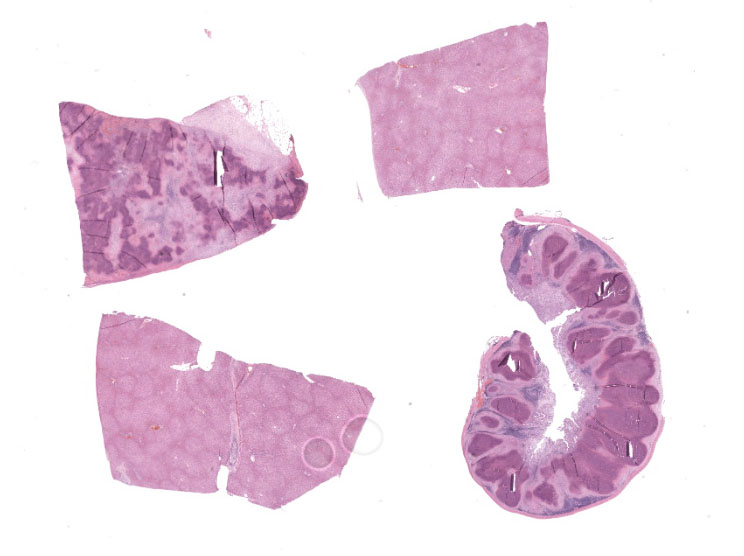
Microscopic Description:
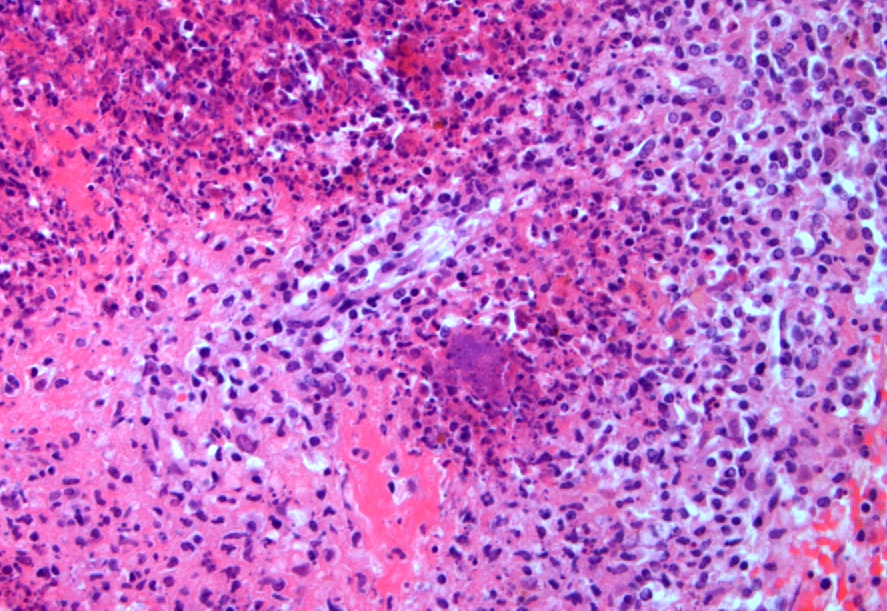
Spleen: Affecting approximately 80% of the examined sections and effacing white and red pulp are large multifocal to coalescing foci of necrosuppurative inflammation characterized by a central, hypereosinophilic area of cellular and karyorrhectic debris (lytic necrosis) admixed with viable and degenerate neutrophils surrounded by numerous macrophages, lymphocytes, and fewer plasma cells. There is an outer layer of fibrous tissue which is infiltrated by lymphocytes, plasma cells, and eosinophils, and often contains scattered to extensive areas of extravasated erythrocytes (hemorrhage) and eosinophilic, fibrillar material (fibrin). Multifocally within and often at the periphery of the areas of necrosis there are colonies, up to 100 µm in diameter, of 1-2 µm amphophilic, gram negative coccobacilli. Scattered throughout the affected and non-affected parenchyma are moderate numbers of macrophages containing intracytoplasmic, golden brown, granular pigment (hemosiderin).
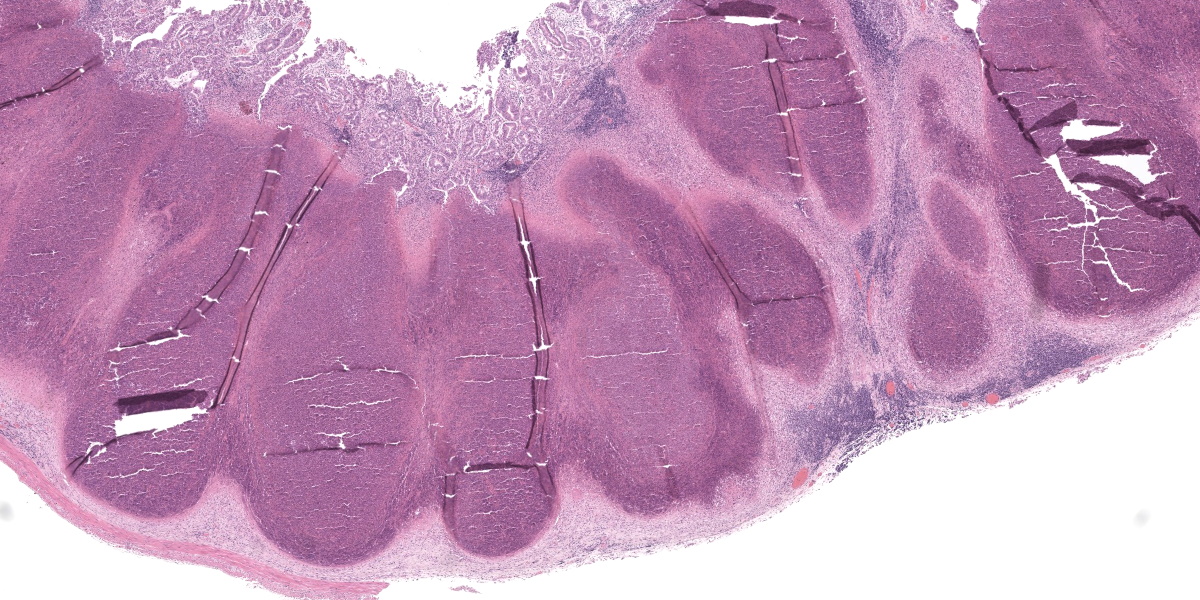
Cecum: In approximately 90-95% of the examined section, the submucosa is markedly effaced and expanded by the presence of multifocal large foci of necrosuppurative inflammation which often extend into the lamina propria. These foci are characterized by a central, area of hypereosinophilic cellular and karyorrhectic debris (lytic necrosis) admixed with mostly degenerate and fewer viable neutrophils, surrounded by numerous macrophages, lymphocytes and fewer plasma cells, multifocal eosinophilic, fibrillar material (fibrin), and by an outer layer of fibrous tissue which is infiltrated by fewer numbers of lymphocytes, plasma cells, and eosinophils. Multifocally within areas of necrosis there are small numbers of scattered, 1-2 µm amphophilic, gram negative coccobacilli. The adjacent mucosal associated lymphoid tissue is multifocally compressed and the remaining submucosa is heavily infiltrated by mostly lymphocytes and fewer plasma cells and macrophages, which occasionally contain black and green, granular intracytoplasmic material. Multifocally within the lamina propria there are mildly to moderately increased numbers of lymphocytes and plasma cells.
Liver: Multifocally, adjacent to portal spaces, there are a few smaller foci of necrosuppurative inflammation with extensive lytic necrosis similar to those described in the spleen and caecum. The portal spaces are often mildly to markedly infiltrated by lymphocytes and plasma cells, which occasionally mildly extend through the limiting plate to the adjacent parenchyma. Small numbers of lymphocytes are often infiltrating the biliary epithelium. Across all examined sections, there are numerous multifocal to coalescing, midzonal to centrilobular areas where hepatocytes are markedly swollen, exhibiting a pale staining cytoplasm arranged in thin strands, or vacuolar change of the cytoplasm (hepatocellular degeneration). Rarely, there is variable occlusion of the lumen of blood vessels by fibrin thrombi with few enmeshed neutrophils and lymphocytes.
Contributor’s Morphologic Diagnoses:
- Spleen: Splenitis, necrosuppurative, subacute, multifocal to coalescing, severe, with intralesional colonies of coccobacilli and microabscesses
- Cecum: Typhlitis, necrosuppurative, subacute, multifocal to coalescing, severe, with intralesional coccobacilli and microabscesses
- Liver: Hepatitis, necrosuppurative, subacute, multifocal to coalescing, mild, with multifocal microabscesses and marked hepatocellular degeneration.
Contributor’s Comment:
Yersiniosis (or pseudotuberculosis) is a disease caused by infection with gram-negative bacteria Yersinia pseudotuberculosis or Yersinia enterocolitica.1,3,14 While susceptible, domestic rabbits are not commonly affected.1 This infection is, however, commonly seen in rodents, birds, non-human primates, ruminants, and other zoo species.2,3,7,11,14
Transmission is thought to occur via the fecal-oral route through ingestion of contaminated food and/or water.1,14 Infection may result either in acute death or development of clinical signs and lesions, as is the case with ruminants and pigs.3,14 In this case, the clinical history and length of duration of clinical signs was unknown; however, the morphology of the lesions identified would suggest a more subacute timeline of infection.
After ingestion, the bacteria colonize the intestinal epithelium M cells, resulting in the destruction of Peyer’s patches and epithelium and the formation of suppurative inflammation and microabscessation in the lamina propria.14 Yersinia spp. virulence requires the presence of a 70-kb virulence plasmid.9,14 This plasmid encodes the production of Yops proteins which inhibit phagocytosis, aiding the bacterial spread to mesenteric lymph nodes and other organs.9,14,15 The bacteria may then disseminate to the liver or systemically through the venous drainage and lymphatic system.14 Typical post-mortem findings include necrotizing hepatitis and splenitis, fibrinonecrotizing enterocolitis, and lymphadenitis.2,3,9,14 On histology, there may be microcolonies of gram-negative coccobacilli and formation of microabscesses.14 It is not possible to differentiate between Y. pseudotuberculosis and Y. enterocolitica based on macroscopic and histological lesions alone.14
As zoonotic agents, Y. pseudotuberculosis and Y. enterocolitis can cause acute gastroenteritis and mesenteric lymphadenitis in humans.8 In Europe, multiple strains of Y. pseudotuberculosis of varying pathogenicity have been identified in wild boars, and pathogenic strains of Y. enterocolitis have been associated with wildlife, suggesting that wildlife species may play an important role as a reservoir for human infections.6,13 While hospitalizations and deaths secondary to these infections have been reported, it is important to differentiate yersiniosis from plague, which is caused by Yersinia pestis and is a World Health Organization-reportable disease with much higher fatality rates.3,8,17
In veterinary medicine, macroscopic findings in Y. pestis infections may include pulmonary, lymph node, and cutaneous/subcutaneous hemorrhages, as well as necrosuppurative lymphadenitis, necrotizing pneumonia, and multifocal abscessation with intralesional gram-negative coccobacilli with a characteristic bipolar, safety-pin-like morphology.3 Geographic location may help include or exclude Y. pestis as a likely/unlikely differential.
The diagnosis of yersiniosis is typically made through identification of microabscesses with microcolonies of coccobacilli on histology and subsequent confirmation with bacterial culture.14 Francisella tularensis infection – tularemia - can cause small foci of caseous granulomas in the liver, spleen and lymph nodes, and could therefore be a potential differential diagnosis for the gross lesions.4 Amongst other lesions, systemic listeriosis, caused by Listeria monocytogenes, can also present with hepatic necrotic foci, and could potentially be a less likely differential; however, gram-positive staining would differentiate this condition from yersiniosis.4
Contributing Institution:
Veterinary Pathology Service
School of Veterinary Medicine and Science
University of Nottingham
https://www.nottingham.ac.uk/vet/
JPC Diagnoses:
- Cecum: Peyer’s patch necrosis, diffuse, with mild, chronic typhlitis.
- Spleen: Splenitis, necrotizing and heterophilic, chronic, diffuse, severe, with coccobacilli.
- Liver: Vacuolar change, centrilobular and midzonal, diffuse.
JPC Comment:
The contributor provides an excellent overview of the typical course of disease in yersiniosis caused by Y. pseudotuberculosis and Y. enterocolitica. These two species are typically associated with a relatively benign gastrointestinal disease rather than the well-described, fulminant syndromes associated with Y. pestis, but the pathogenetic mechanisms of the enteric disease caused these species remain incompletely understood.
As the contributor notes, Y. pseudotuberculosis and Y. enterocolitica cross the mucosa through the M cells of Peyer’s patches; once in the mucosa, they are engulfed by macrophages, where they survive and are trafficked to the lymph nodes and beyond.12 A key virulence factor, shared by all three pathogenic Yersinia species, is a type 3 secretion system, similar to that found in Salmonella species, that allows the organism to inject effector proteins into the host cell cytoplasm.12 In Yersinia species, these effector proteins are termed Yersinia outer proteins (“Yops”). Yops are injected into macrophages and neutrophils and interfere with phagocytosis and the production of reactive oxygen species, ensuring bacterial survival and dissemination.12 Yops come in at least six different varieties – YopE, YopM, YopO, YopT, YopP, and YopH – each with its own pernicious bag of tricks aimed at frustrating the host’s immune system.5 For instance, YopP blocks pro-inflammatory NF-kappaB and MAPK-signaling pathways, and YopM is thought to block the inflammasome by binding caspase-1.5 The end result of all of this molecular subterfuge is bacterial persistence and disease.
Birds and rodents are throught to be the principal reservoirs of Yersinia pseudotuberculosis, which causes enteric, often subclinical infection in many wild and domestic animals.12,16 Enteric yersiniosis is relatively common in young deer and lambs, where disease is characterized by profuse watery diarrhea which can be rapidly fatal if untreated; clinically similar but less severe disease can be seen in young ruminants.12 The septicemic form of the disease, often called pseudotuberculosis, occurs most commonly in laboratory rodents and aviary birds.12 Finally, sporadic abortion caused by Y. pseudotuberculosis has been reported in cattle, sheep, and goats.12
Despite the canonical association of Y. pseudotuberculosis with enteritis, a recently reported outbreak in African lions was notable for its presentation, which was characterized by respiratory distress and pulmonary bacterial colonization without gastrointestinal lesions.16 Also notable was the unusual pleomorphic, filamentous morphology of the bacteria, confirmed to be Y. pseudotuberculosis via culture.16 This filamentous morphology has been previously described in Y. pseudotuberculosis infections in squirrel monkeys and is thought to be a bacterial adaptation to the use of antibiotics.16
Our moderator this week was Dr. Patti Pesavento, Department Chair and Professor of Pathology, Microbiology, and Immunology at the University of California Davis College of Veterinary Medicine. Dr. Pesavento began discussion of this case by noting that low-magnification evaluation is invaluable in this slide, which contains four tissues, some of which contain flashy lesions with remarkable distributions and tinctorial differences. Dr. Pesavento cautioned against getting bogged down looking at every micrometer of tissue and exhorted residents to use low magnification to spot areas of interest, take closer looks at those particular areas, and then hop back to subgross magnification for further investigation.
Conference participants found tissue identification on the section of alimentary tract to be somewhat difficult and discussed possible locations, including the vermiform appendix, he cecal tonsil, the ilem, or the sacculus rotundus. Participants felt they could not be definitive based on the examined section alone due to the complete obliteration of tissue architecture both in this tissue and in the spleen. Participants also noted the presence of substantial fibrosis indicating that, despite the apparent fulminant nature of the lesions in the spleen and gastrointestinal tract, the process was likely chronic. Chronicity could also explain the lack of large cloud-like bacterial colonies which would be expected of Yersinia spp., the “Y” in the “STACY” mnemonic used to denote large colony-forming bacteria (the others being Staphylococcus, Streptococcus Actinomyes, Actinobacillus, Corynebacterium, Clostridium, and Trueperella).
Participants marveled at the liver, which had no right to look as great as it did. Given that Y. pseudotuberculosis enters from the gut, the liver receives the brunt of the bacterial onslaught. Dr. Pesavento noted that the liver does an admirable and largely unsung job of clearing bacteria from the portal blood and, considering the destruction noted in the spleen and gut, the relatively normal looking liver is a testament to the its effective immunologic vigilance. Some participants noted the mild periportal lymphoplasmacytic hepatitis, which is a fairly typical finding in rabbits, and thought it unrelated to the bacterial infection. Participants did appreciate a mild lipid vacuolation that seemed concentrated in the centrilobular and midzonal areas, and felt that this was the most believable lesion present in the liver.
Once participants were thoroughly unimpressed with the histologic appearance of the liver, Dr. Pesavento pulled a proverbial rabbit out of the hat, wowing the crowd with a reticulin stain. Reticulin, a connective tissue composed of type III collagen, lines the spaces of Disse. When visualized with a reticulin silver stain, the patency of these fibers can be assessed to determine the integrity of the hepatic cord and sinusoidal architecture. In this case, the seemingly bland appearance of the liver belied the fact that the entire reticulin network was completely destroyed.
Participants felt that the cecal tissue should get pride of place in the morphologic diagnosis given the striking necrosis. Participants could not convincingly identify coccobacilli in the examined cecal section, but were convinced of their presence in the spleen. The remarkable liver, despite its lack of underlying reticulin network, was felt to be largely normal in the examined section, with no areas of necrosis or identifiable bacteria. Participants felt, however, that the zonal distribution of the vacuolar change was a believable, perhaps sentinel lesion, and deserved to be included in the diagnosis.
References:
- Barthold SW, Griffey SM, Percy DH. Rabbit. In: Pathology of Laboratory Rodents and Rabbits. John Wiley & Sons; 2016:253-324.
- Ceccolini ME, Macgregor SK, Spiro S, Irving J, Hedley J, Guthrie A. Yersinia pseudotuberculosis infections in primates, artiodactyls, and birds within a zoological facility in the United Kingdom. J Zoo Wildl Med. 2020;51(3):527-538.
- Delaney MA, Treuting PM, Rothenburger JL. Rodentia. In: Pathology of Wildlife and Zoo Animals. Elsevier 2018:499-515.
- Delaney MA, Treuting PM, Rothenburger JL. Lagomorpha. In: Pathology of Wildlife and Zoo Animals. Elsevier 2018:481-498.
- Grabowski B, Schmidt MA, Ruter C. Immunomodulatory Yersinia outer proteins (Yops) – useful tools for bacteria and humans alike. Virulence. 2017;8(7): 1124-1147.
- Le Guern AS, Martin L, Savin C, Carniel E. Yersiniosis in France: Overview and potential sources of infection. Int J Infect Dis. 2016;46:1-7.
- Hammerl JA, Barac A, Bienert A, et al. Birds kept in the German zoo “Tierpark Berlin” are a common source for poly-valent Yersinia pseudotuberculosis pha-ges. Front Microbiol. 2022;12: 634289.
- Long C, Jones TF, Vugia DJ, et al. Yersinia pseudotuberculosis and Y. enterocolitica Infections, FoodNet, 1996–2007. J Infect Dis. 2010;16(3):566-567.
- Najdenski H, Vesselinova A, Golkocheva E, Garbom S, Wolf-Watz H. Experimental infections with wild and mutant Yersinia pseudotuberculosis strains in rabbits. J Vet Med Ser B. 2003;50(6):280-288.
- Nakamura S, et al. Aberrant form of Yersinia pseudotuberculosis as sphero-plasts and filaments in yersiniosis in squirrel monkeys. Vet Pathol. 2012;52: 393-396.
- Pereira M, Stidworthy MF, Denk D, Spiro S, Guthrie A, Patterson S. A retrospective study of morbidity and mortality identified at postmortem examination of captive langurs (Trachypithecus spp) from six United Kingdom zoological institutions: A 19-year review. J Zoo Wildl Med. 2021;52 (4):1123-1134.
- Quinn PJ, Markey BK, Leonard FC, FitzPatrick ES, Fanning, S, Hartigan PJ. Veterinary Microbiology and Microbial Disease. Wiley-Blackwell;2011:280-282.
- Reinhardt M, Hammerl JA, Kunz K, Barac A, Nöckler K, Hertwig S. Yersinia pseudo-tuberculosis prevalence and diversity in wild boars in northeast Germany. Appl Environ Microbiol. 2018;84(18):1-10.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy & Palmer’s Pathology of Domestic Animals. 6th ed. Vol 2. Elsevier 2016.
- Visser LG, Annema A, Van Furth R. Role of Yops in inhibition of phagocytosis and killing of opsonized Yersinia entero-colitica by human granulocytes. Infect Immun. 1995;63(7):2570-2575.
- Womble M, Cabot ML, HarrisonT, Terumi Negrao Watanabe T. Outbreak in African lions of Yersinia pseudo tuber-culosis infection, with aberrant bacterial morphology. J Vet Diag Invest. 2022; 34(2):334-338.
- World Health Organization. Plague. 2022:1-5. Available at https://www.who. int/news-room/fact-sheets/detail/plague; accessed on 05/23/23.