Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 14
9 January, 2019
CASE II: S1603556 (JPC 4084299).
Signalment: A 1-year-old Quarter horse filly
History: The filly had a history of 10-day lethargy. The owner was suspicious of a rattlesnake bite.
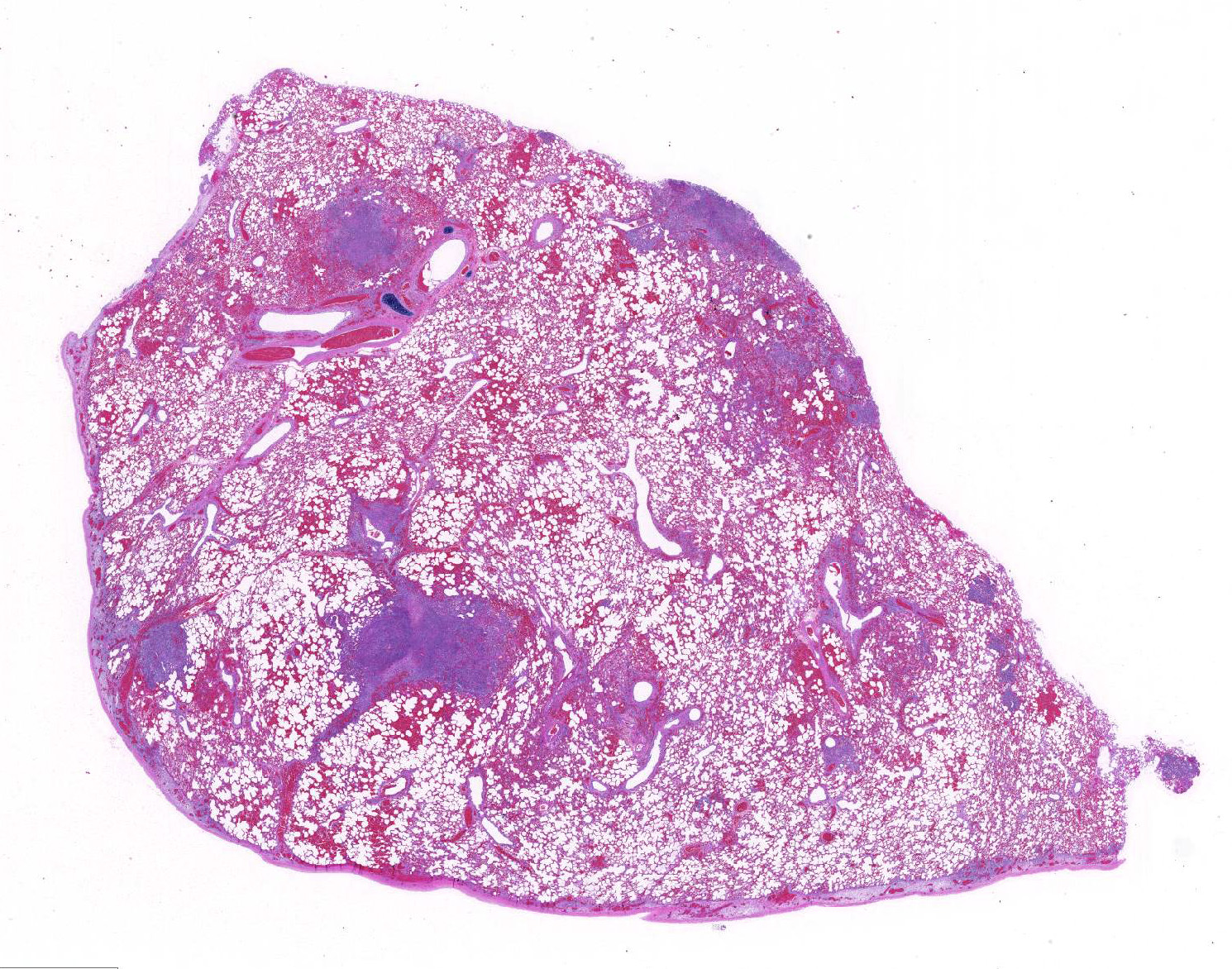
Gross Pathology: The lip was diffusely thickened with edema. Both submandibular lymph node were enlarged, firm and dark red. Bilaterally, the parietal pleura had multiple petechiae and ecchymoses. Multifocally in both lungs there were numerous white, firm nodules, approximately 2 to 3mm in diameter, surrounded by a dark red halo. The parenchyma between these nodules was dark red and firm. The pericardium, mainly in proximity to the coronary vessels and paragonal septum, also showed several multiple petechiae and ecchymoses. The kidneys had several multifocal white, firm cortical spots of approximately 1 to 3mm in diameter. The cortex was diffusely pale. The mesentery had multifocal random white firm nodules of approximately 3 to 5 mm in diameter surrounded by a dark red halo.
Laboratory results: No bacterial pathogens were isolated form liver, lung, spleen and submandibular lymph node on aerobic culture.
Microscopic Description:
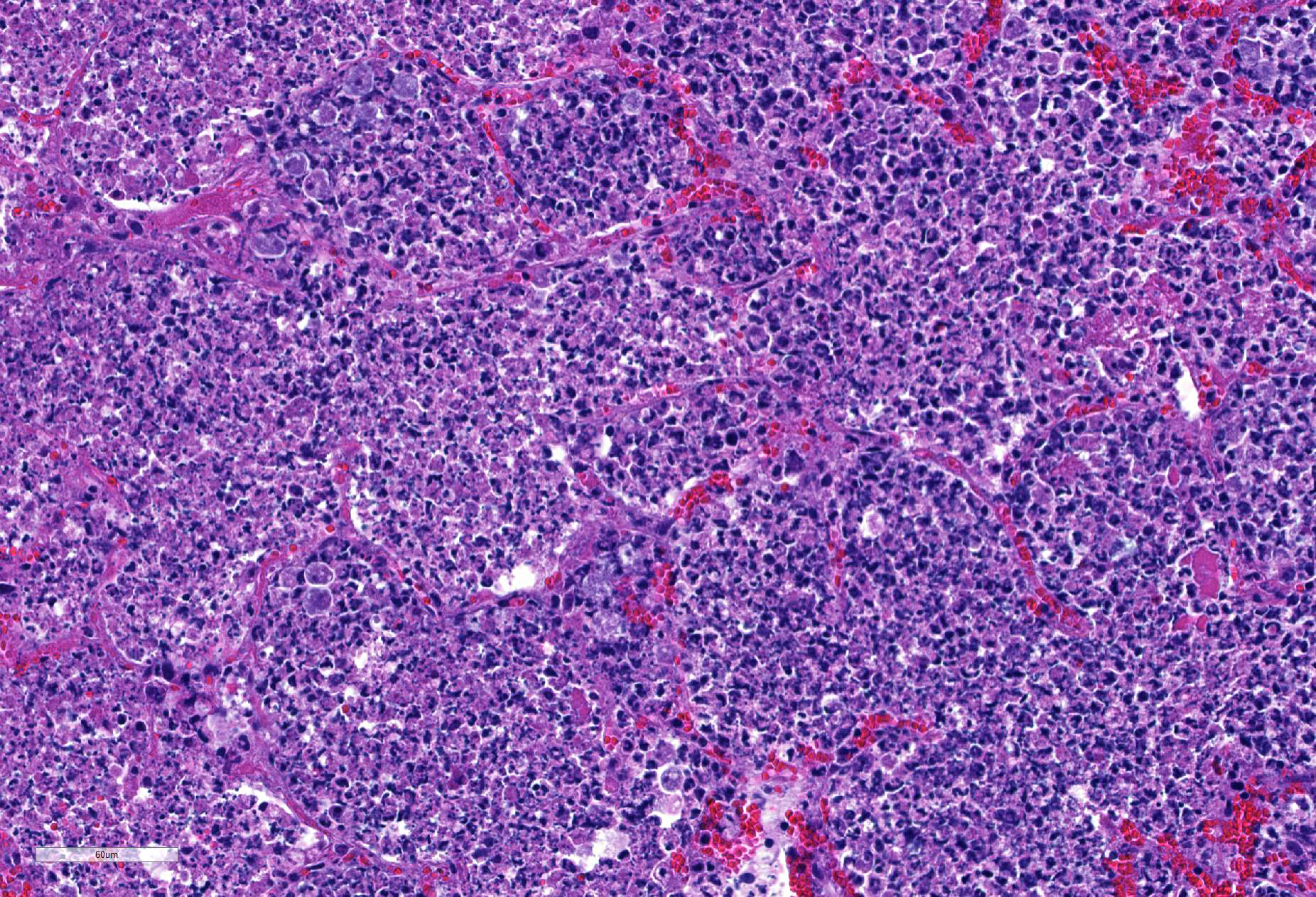
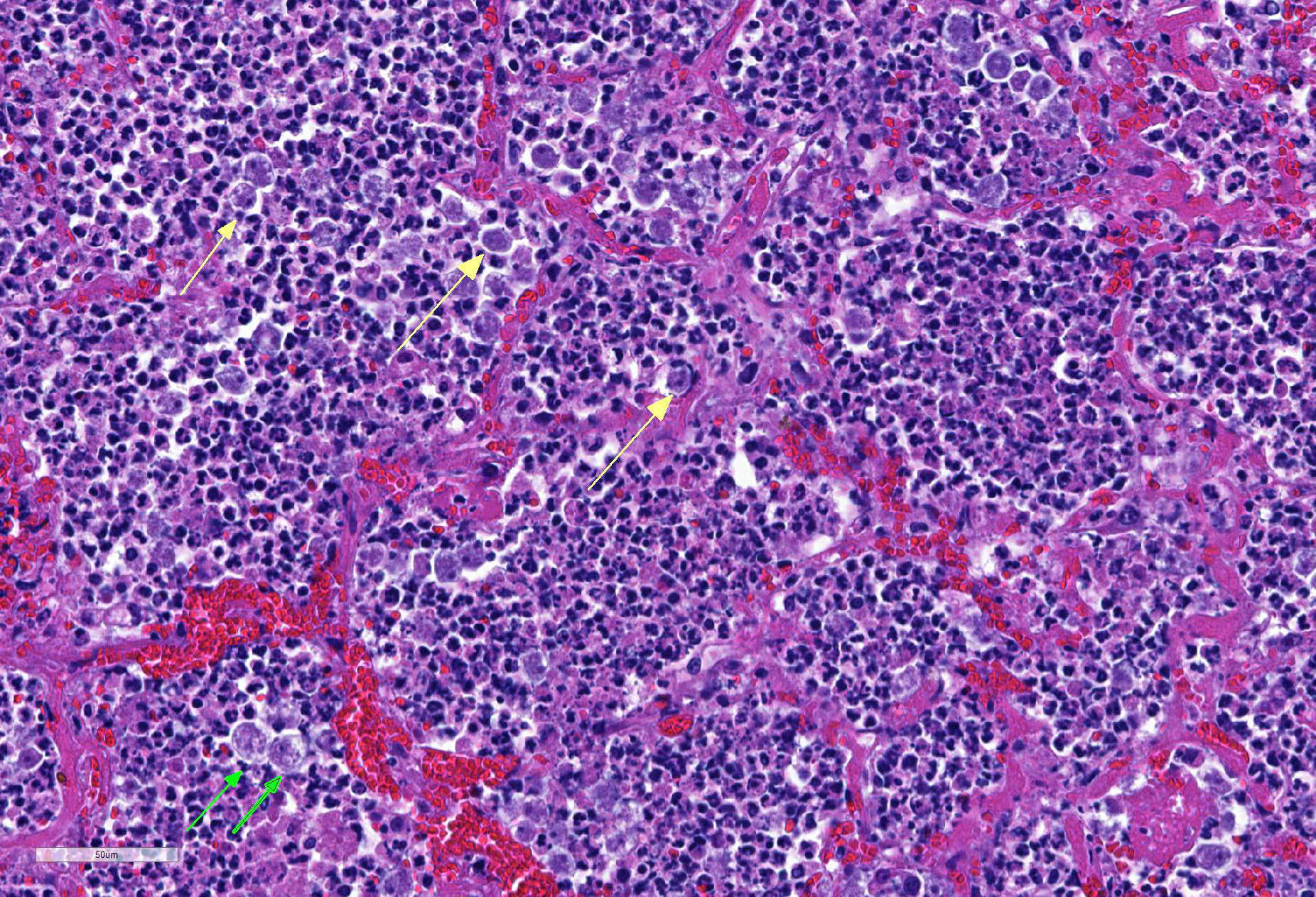
Lung: centering in small caliber vessels and extending into the adjacent alveolar spaces and septa, there is a multifocal and random inflammatory infiltrate composed by large numbers of viable and degenerated neutrophils, fewer histiocytes and occasional multinucleated giant cells that are admixed with basophilic cellular debris and several rounded eosinophilic protozoa of approximately 15 to 20 µm in diameter, with an eccentric single round nucleus. In addition, there are numerous fibrin thrombi and segments of alveolar necrosis. A few blood vessels display transmural necrosis, with fibrinoid degeneration and the presence of pyknotic debris. The remaining parenchyma shows moderate congestion, occasional small hemorrhages and multiple focal areas of intra-alveolar edema.
Contributor’s Morphologic Diagnosis:
Embolic pneumonia, necrosuppurative, severe, multifocal, with numerous trophozoites.
Etiology: Acanthamoeba sp.
Additional Findings: Trophozoites were immunolabeled for Acanthamoeba sp. in the lung, kidney and lymph node via immunohistochemistry test. In addition, a lymphohistiocytic to granulomatous interstitial nephritis, myocardial necrosis and mineralization, focal suppurative myositis; moderate to severe suppurative, lymphoplasmacytic and histiocytic, necrotizing peritonitis, gastritis and hepatitis, with protozoa consistent with Acanthamoeba sp. were also observed.
Contributor’s Comment: Acanthamoeba sp. are ubiquitous free-living amoebae distributed in the environment.2,3 Disease produced by these amoebae have been described in cows, dogs, pigs, rabbits, pigeons, sheep, reptiles, fish, turkeys, rhesus macaque and toucans.7,8 Amoebic infection in horses is very rare and it has been reported in Southern California, USA, causing pneumonia, meningoencephalitis4 or systemic infections, and in placentitis in the state of New South Wales, Australia1. The reason for these presentations is still not clear. Amoebas can be easily missed in histology sections because of the morphology of their trophozoites, which resemble macrophages1. Reports of human infection with Acanthamoeba sp. have traditionally been limited to cases of systemic disease in the immunocompromised, granulomatous encephalitis, keratitis and cutaneous and sinus lesions. 2
Acanthamoeba has two stages in its life cycle, a vegetative trophozoite stage with a diameter of 13-23 µm and dormant cyst stage of 13-23 µm. During the trophozoite stage, Acanthamoeba feeds on organic particles as well as other microbes and divides mitotically under optimal conditions. 5 It has been proposed as a reservoir of several microorganisms as Legionella pneumophila, Pseudomonas aeruginosa, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Klebsiella spp. and Streptococcus pneumoniae. 2
Amoebae can be cultured from lesions by special methods, which are not available in most diagnostic laboratories. Speciation may be accomplished by fluorescent antibody testing and immunohistochemistry.3
Information on treating topical and systemic amoebic infections in animals is lacking. 3
Contributing Institution:
California Animal Health and Food Safety Lab. San Bernardino Branch. University of California, Davis
JPC Diagnosis: Lung: Pneumonia, necrosuppurative, multifocal to coalescing, moderate, with numerous extracellular amebic trophozoites.
JPC Comment: Free-living ameba are ubiquitous protozoans in the environment, of which four generally are considered pathogenic for humans and animals: Acanthamoeba, Balamuthia, Naegleria, and Sappinia.1
Acanthamoeba appear to be most often associated with disease in humans and animals, with 18 distinct genotypes based on nuclear small-subunit ribosomal DNA rather than morphology. The most common condition associated with infection in humans is a chronic keratitis, seen in immunocompetent patients associated with improper handling of contact lenses, exposure to contaminated water, or trauma. Risk factors of contact lens users include the use of all-in-one solutions, showering while wearing contact lenses, and poor contact lens hygiene.5 Granulomatous amebic encephalitis is a well-documented syndrome in humans resulting from hematogenous spread, often from the lower respiratory tract or skin lesions. It shows a chronic fatal progression with luckily only 150 documented cases worldwide.5 Due to its hematogenous origins, areas of granulomatous inflammation are seen throughout all parts of the brain. Cutaneous acanthamoebiasis is also an uncommon opportunistic condition primarily seen in immunosuppressed patients, resulting in erythematous sores and skin ulcers.1
Primary amebic meningoencephalitis (PAM) is another rare fatal disease in humans cause by Naegleria fowleri. This condition generally occurs in healthy children and adults swimming or bathing in in warm freshwater ponds. Infective amebae migrate along olfactory nerves from the nose to the brain; fatal infection proceeds rapidly and is almost always fatal. Due to this unique entry portal, areas of lytic necrosis are clustered at the base of the brain; hypothalamus, pons, and occasionally seen in posterior areas such as the medulla oblongata.
Balamuthia mandrillaris is also a cause of granulomatous amebic encephalitis which ranges in duration between Acanthamoeba and Naegleria, which usually results from hematogenous spread from soil-contaminated wounds.
Other species of free-living amoeba which may have been identified in cases of keratitis include Hartmanella, Vahlkamphiae, and Allovahlkampfiae spelae.1
As the contributor mentioned above, free-living amebae may act as vectors for a wide range of pathogenic bacilli. They may also act as hosts for a range of viruses, including coxsackieviruses and adenoviridae pathogenic for humans. Other viruses, the so-called giant viruses, may act as endocytobionts, including representatives of the Mimi-, Moumo- and Megaviridae, as well as Pandoviridae. Co-cultivation in amebae has been of great benefit in the eventual isolation of these putative human pathogens.
The moderator and participants could not definitively identify cysts in tissues although PAS and GMS were run, they were of no help in identifying cysts. Megakaryocytes were identified in the alveolar capillaries. The moderator noted that they can normally be found there as the lungs are a site of thrombopoiesis and as such can serve as reservoirs for platelet production and release them in response to various stimuli.9 Finally, the moderator reviewed the various types of free-living amebae, and participants discussed the associated syndromes in humans and animals.
References:
- Balczun C, Scheid PL. Free-living amoebae as hosts for and vectors of intracellular microorganisms with public health significance. Viruses 2017; 9:^%, doi 10-3390/v9040065.
- Begg A., Todhunter, K., Donahoe, S, Krockenberger, M., Slapeta J. Severe amoebic placentitis in a horse caused by an Acanthamoeba hatchetti isolate identified using next generation sequencing. Journal of clinical microbiology. 3101-3104. 2014
- Bradbury R S, French L.P., Blizzard L. Prevalence of Acanthamoeba spp in Tasmanian intensive care clinical specimen. Journal of Hospital Infection 86, 178-181. 2014.
- Greene C.E. Infectious diseases of the dog and cat. 802-804. Fourth edition. 2012
- Krol-Turminska K, Olendar A. Human infections caused by free-living amoebae. Ann Agric Env Med 2016 24(2):240-260
- Kinde, H., Read D.H.,Daft, B., Manzer, M., Nordhausen R., Kelly D., Fuerst P.A., Booton G., Visvesvara G.S. Infections caused by pathogenic free-living amebas (Balamuthia mandrillaris and Acanthamoeba sp.) in horses. J Vet Diagn Invest 19:317-322. 2007.
- Siddiqui R and Khan N A. Biology and pathogenesis of Acanthamoeba. Review. Parasites and vectors. 5:6. 2012.
- Westmoreland S.V., Rosen J., MacKey J., Romsey C., Xia D.L., Visvesvera G.S., Mansfield K.G.. Necrotizing Meningoencephalitis and Pneumonitis in a Simian Immunodeficiency Virus-Infected Rhesus Macaque due to Acanthamoeba. Veterinary Pathology. July 2004. vol 41 398-404.
- Weyrich AS, Zimmerman GA. Platelets in lung biology. Annu Rev Physiol. 2013;75:569-591.