WSC 22-23
Conference 17
Case IV:
Signalment:
7-year-old male Alaskan Malamute dog
History:
A 7-year-old male Alaskan Malamute dog was presented with non-specific clinical signs (weight loss, apathy and anorexia). Ultrasonography and CT scan revealed the presence of a large mass in the abdominal cavity and multiple smaller masses in the liver. Biopsies of the masses were taken, and a pyogranulomatous hepatitis and lymphadenitis was diagnosed histologically. GRAM, Ziehl-Neelsen (ZN), Grocott and PAS stains did not reveal bacterial or fungal aetiology. Negative results were obtained by PCR for Leishmania spp., Bartonella spp., atypical Mycobacteria, Neospora caninum, Toxoplasma gondii and Cryptosporidium spp. Because of the poor prognosis the dog was euthanatized.
Gross Pathology:
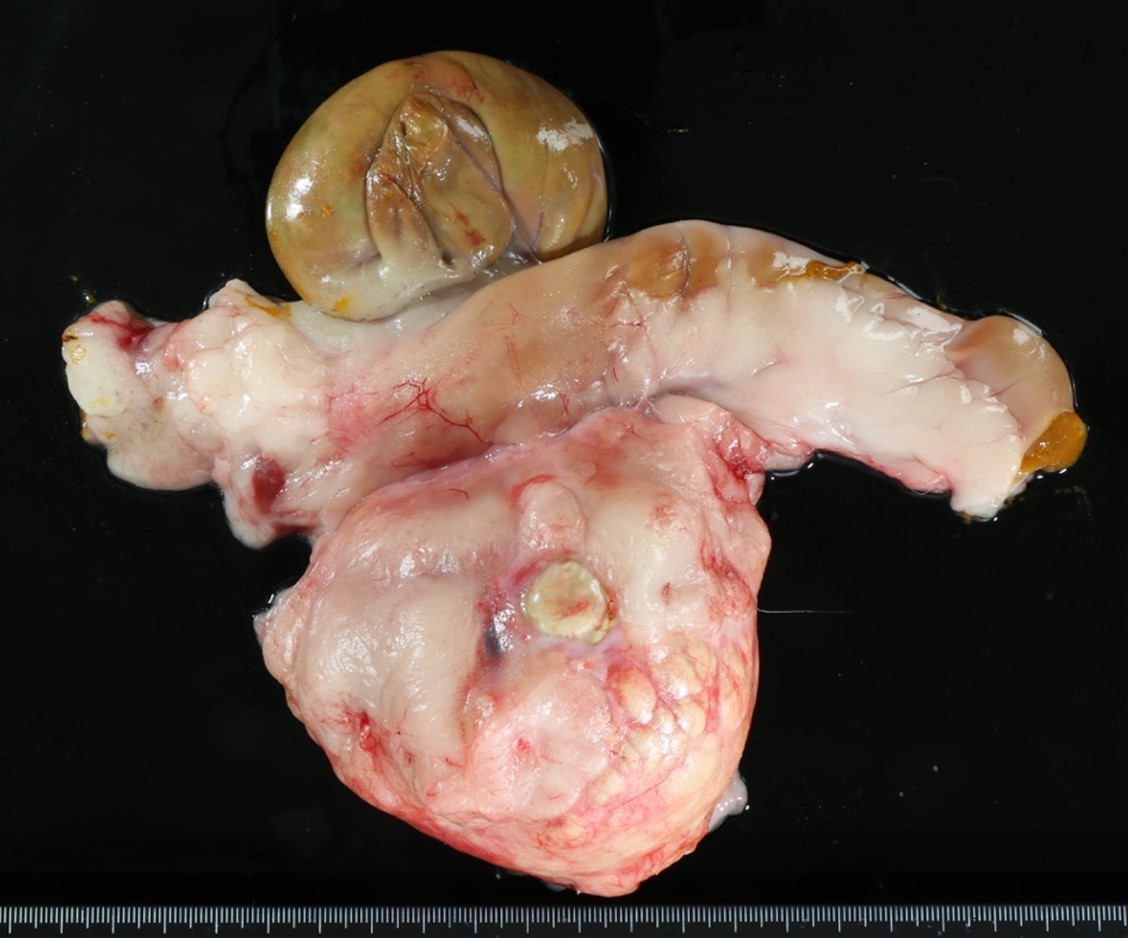
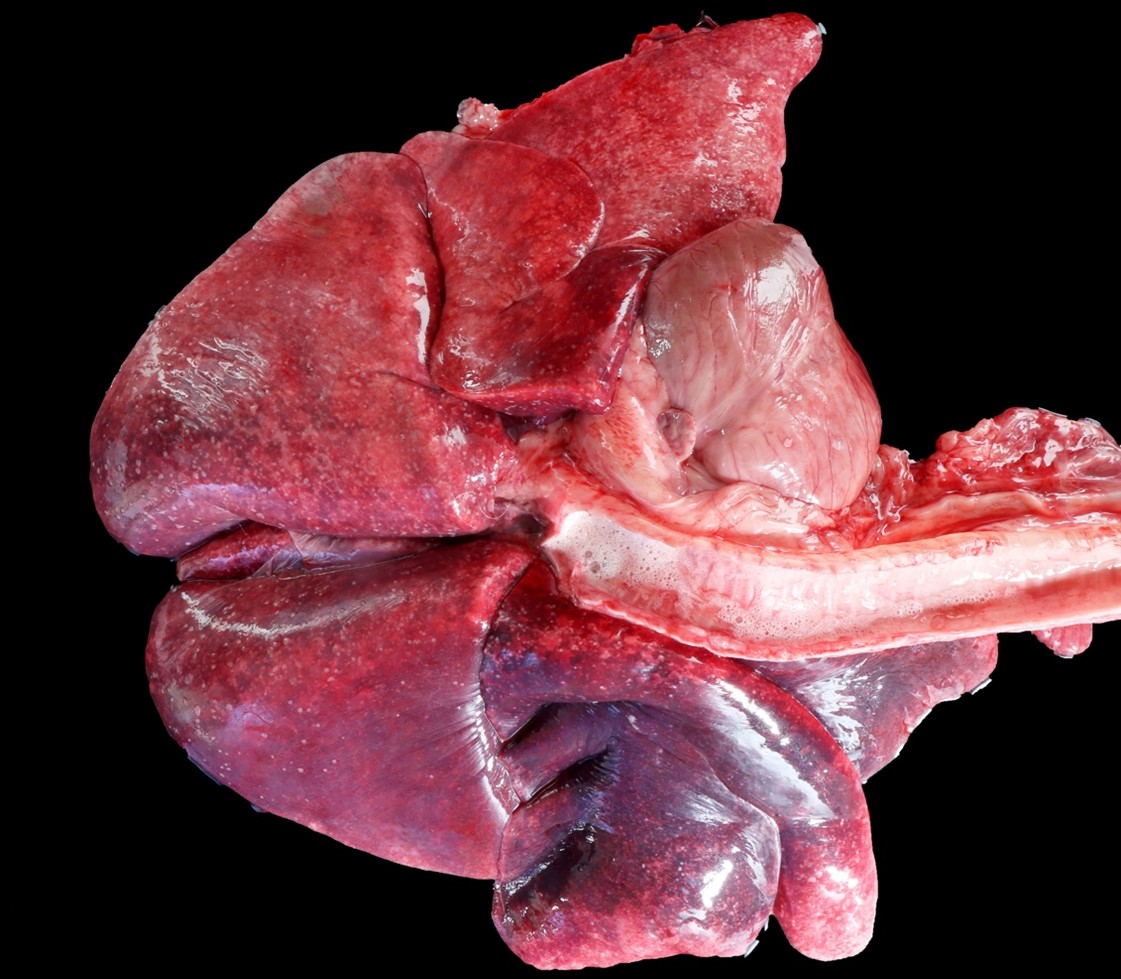
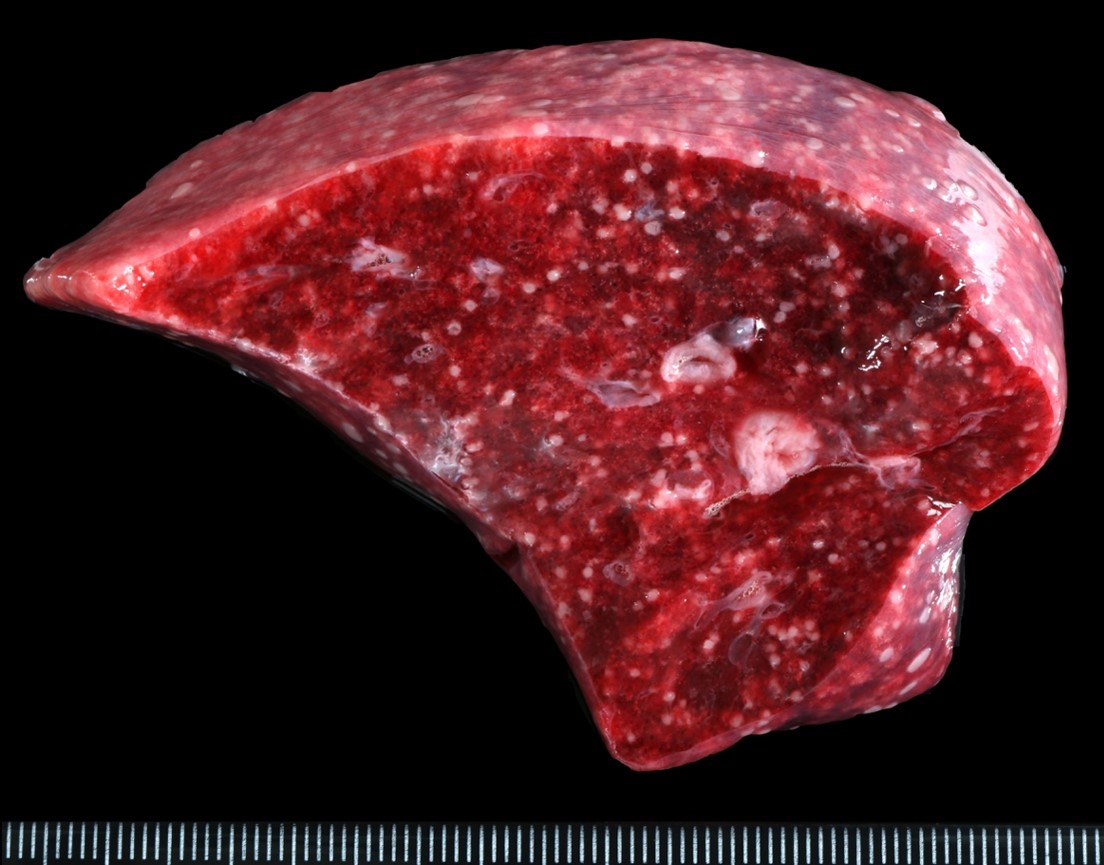
At postmortem examination, the dog showed loss of body condition. The mesenteric lymph node was replaced by a soft, encapsulated mass of about 15 cm. in diameter, with a central area of caseous necrosis. Also, multiple, randomly distributed granulomas of 0.2 to 0.5 cm in diameter were seen along the serosa and in the parenchyma of lung, heart, liver, kidneys, omentum and brain
Laboratory Results:
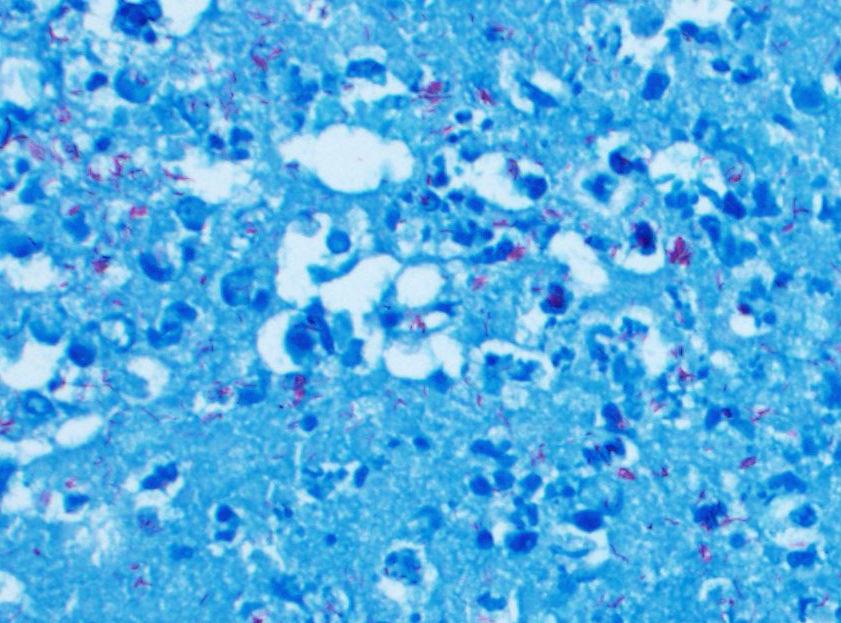
Postmortem: Additional ZN staining were performed on several tissues taken at necropsy. ZN stain showed acid-fast bacilli in the necrotic center of granulomas and also within macrophages. Frozen samples preserved at necropsy were submitted for mycobacterial investigation. Direct PCR for the Mycobacterium tuberculosis complex (MTC) yielded a positive result, and a mycobacteria belonging to the MTC was cultured. The isolated mycobacteria was identified as Mycobacterium tuberculosis by spoligotyping.
Microscopic Description:
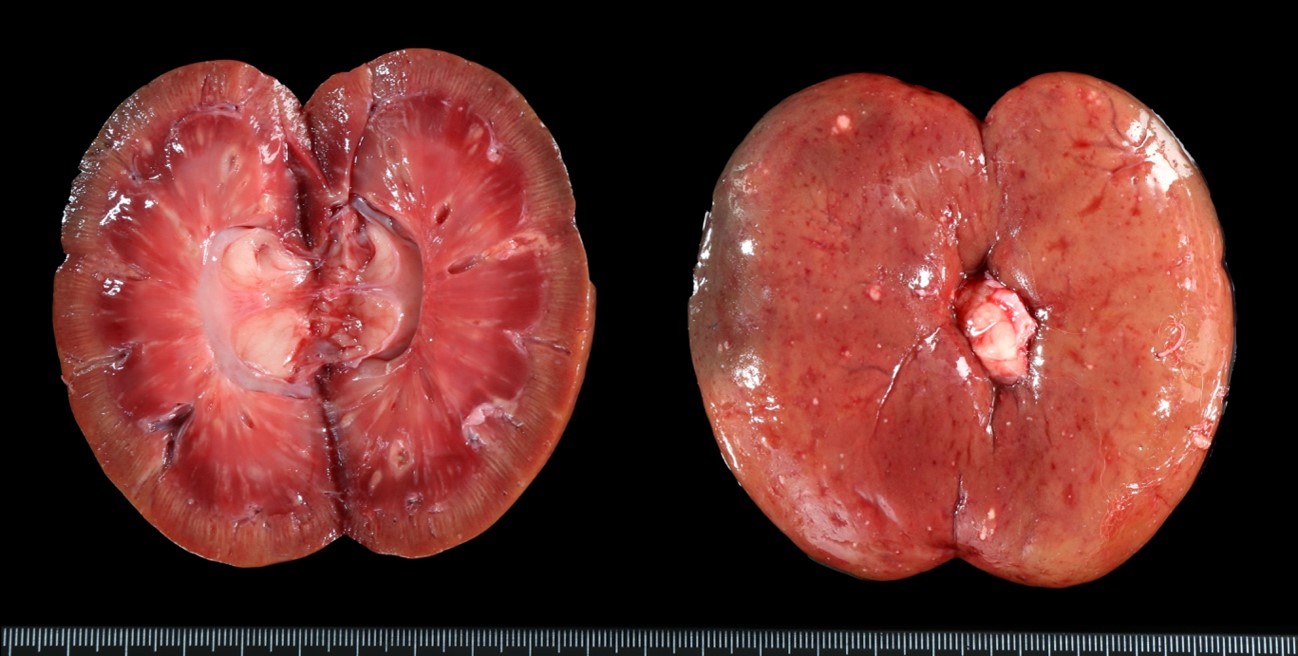
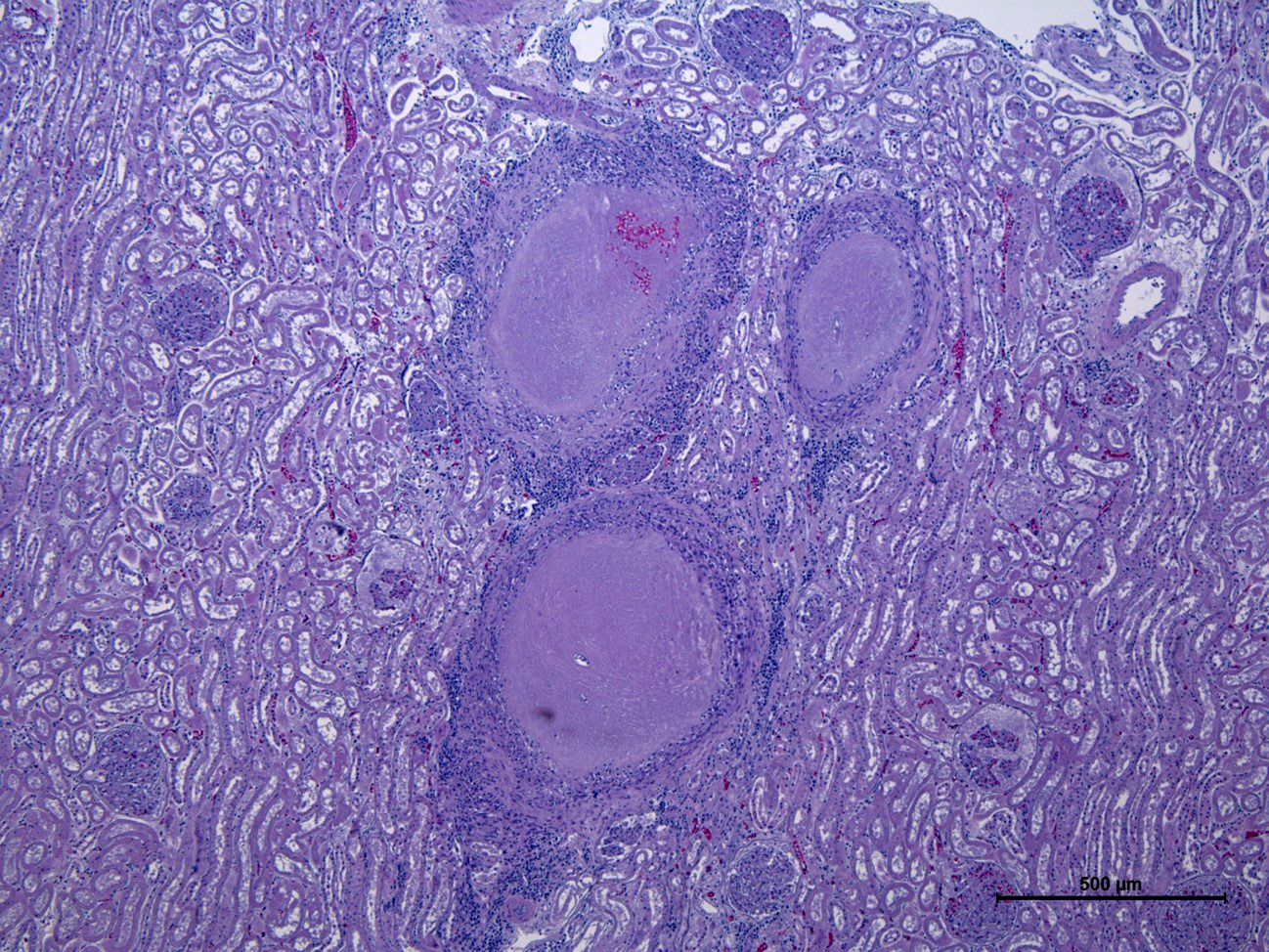
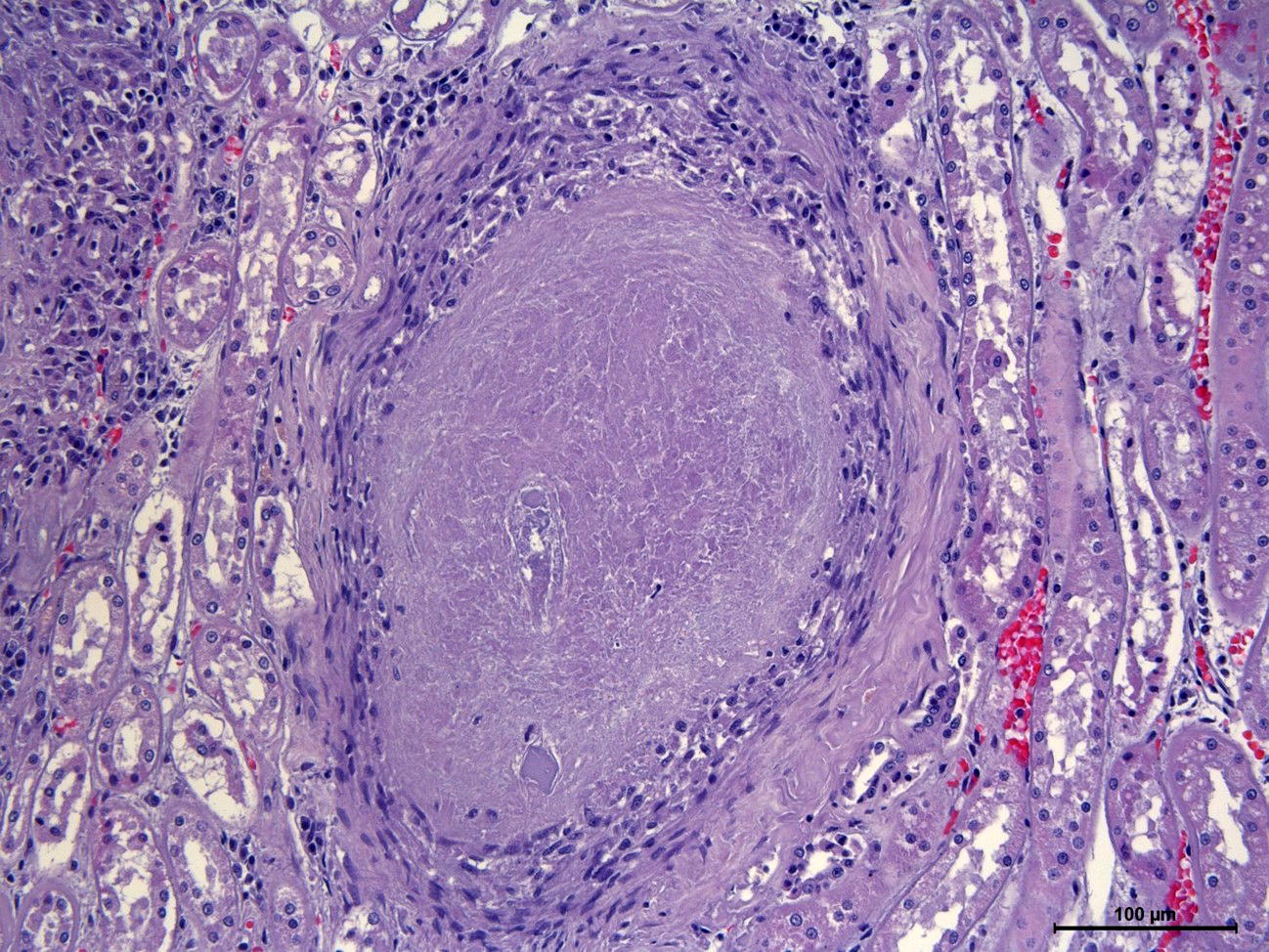
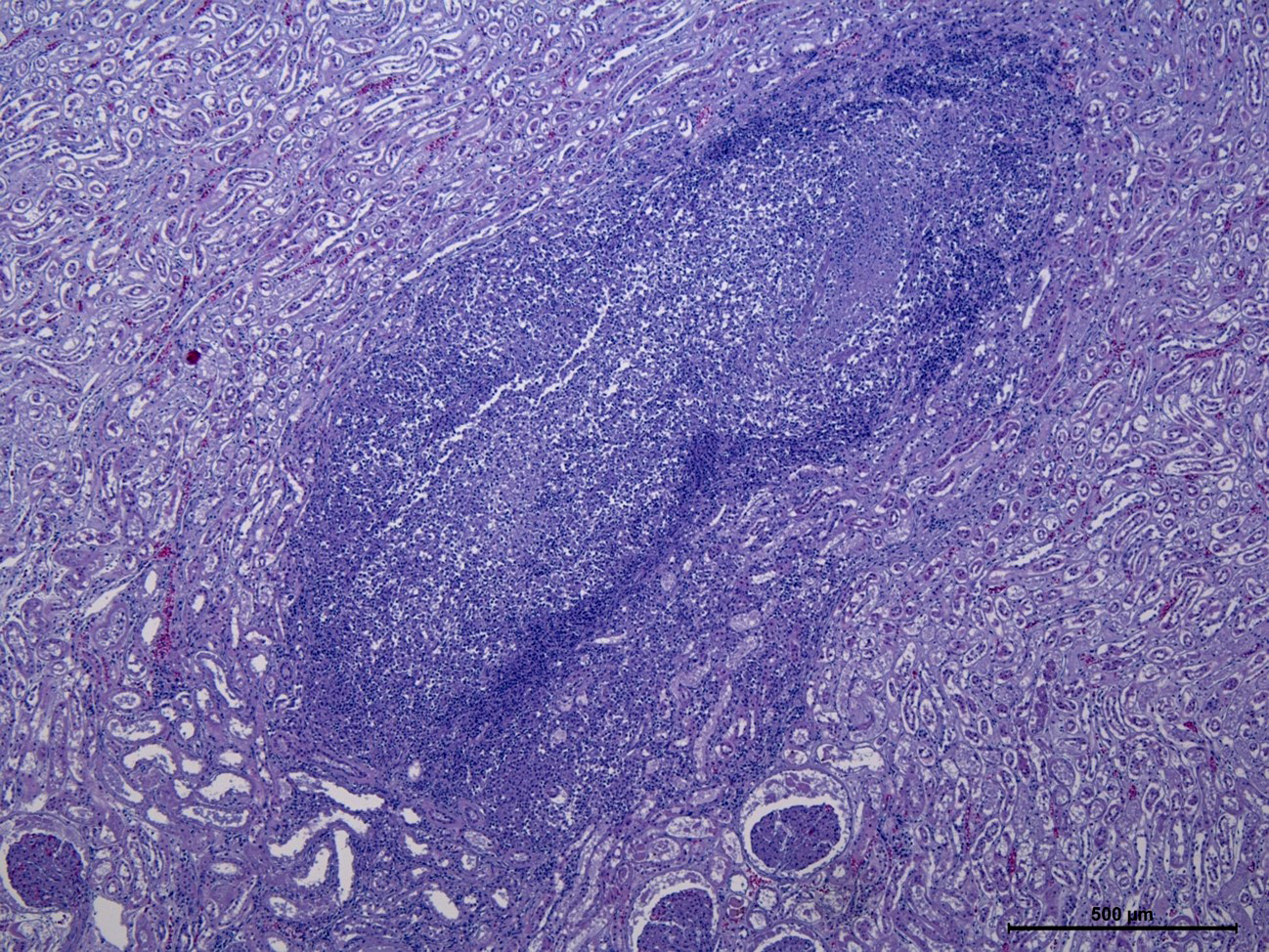
Kidney: Multifocal to coalescing, variably size (0.2 to 0.5 cm in diameter) granulomatous-necrotizing nodular lesions scattered throughout the cortex, medulla and to a lesser extent pelvis, are observed replacing approximately 20-25% of the evaluated parenchyma. The center of some of these nodules is composed of moderate amount of acellular and amorphous hypereosinophilic necrotic material admixed with cellular debris, kariorexis and karyolysis (lytic necrosis). Depending on the section assessed, occasional necrotic glomeruli and tubules are trapped within the necrotic debris also with mild deposition of granular and basophilic mineral material (dystrophic mineralization). These nodules are demarcated by mild to moderate macrophagic inflammation, mainly epithelioid cells, with scarce multinucleated giant cells (Langhans type), surrounded by a thin capsule of mature connective tissue (fibrosis) at the periphery (Granuloma). Multifocally, other non-encapsulated granulomas are also observed and are composed of moderate amount of viable and degenerated neutrophils and macrophages without obvious areas of lytic necrosis in the center. Acid-fast bacilli were observed in the cytoplasm of macrophages and free within the necrotic material with ZN staining. Diffusely, the glomeruli of non-affected parenchyma showed moderate thickening of the glomerular Bowman capsule and basement membranes due to the presence of acellular and amorphous eosinophilic material.
Contributor’s Morphologic Diagnoses:
Nephritis, granulomatous and necrotizing, multifocal to coalescing, severe, chronic.
Contributor’s Comment:
Tuberculosis (TB) is a zoonotic disease caused by mycobacteria of the Mycobacterium tuberculosis complex (MTC) in a broad range of mammalian hosts. MTC comprises M. tuberculosis, M. bovis, M. africanum, M. microti, M. canetti, M. caprae, and M. pinnipedii.3,17 Though a significant progress has been made toward the elimination of tuberculosis from humans, this disease remains an important health, social and economic problem. 1,15
M. tuberculosis and M. bovis occur most frequently in their respective hosts, but may infect many other species. Humans are the only reservoir host for M. tuberculosis; however, this bacterium has a wide host range, including dogs, cats, pigs, cattle, captive monkeys, psittacine birds, fish, reptiles and marine animals.2,7,17,18
Dogs and cats have been pointed as potential carriers and as a possible source of tuberculous infection to other species.1 The susceptibility of dogs to infection with M. tuberculosis is still a matter of debate. Malin (1940) was the ?rst who reported tuberculosis in dogs and their owners. Later on, tuberculosis in dogs was linked to the disease in humans and cattle.1,14 Studies on dogs being in contact with people su?ering from tuberculosis show high levels of M. tuberculosis isolated from otherwise healthy-looking animals and, in some cases, dogs infected with M. tuberculosis demonstrated the full clinical picture of the disease.1
Canine TB has been caused by M. tuberculosis, M. bovis, and M. microti. Although dogs are less susceptible to M. tuberculosis than to the other tuberculous mycobacteria, they can become infected after prolonged exposition to infected human respiratory secretions.1,4,15,16 Thus, M. tuberculosis infection in dogs is considered an anthropozoonosis.7,11 Therefore, the incidence of canine TB is closely related to the incidence of human TB, being higher in urban areas, where the human patients are concentrated, or in developing countries.1,7,17 Middle Africa and Southern Asia seem to be the parts of the world where human TB is most prevalent.16 Evidence indicates that many companion animals infected with M. tuberculosis had a history of living in an environment with humans infected by TB, or that the animals had intimate contact with owners affected by TB.11 Genotypic comparison of mycobacteria recovered from owners and dogs may con?rm the similarity of strains that infected household members and/or their pet.5,11
The inhalation of aerosolized droplets from humans appears to be the primary route of canine TB infections.13 In addition, cutaneous and ingestion of contaminated food or sputum are well-recognized sources of M. tuberculosis transmission from humans to dogs.1,11,13
Although no clear canine-to-human M. tuberculosis transmission has been documented, dogs can discharge organism in the sputum leading to elimination of M. tuberculosis into the home environment.1,11,17 In addition, experimental infection results led to the assumption that the transmission of M. tuberculosis between infected and healthy dogs kept in close contact is possible, suggesting that naturally infected dogs may be a continuous source of infection for humans and other animals.1,11
Natural TB in companion animals is most commonly a subclinical disease. TB-infected dogs with less than two years old rarely exhibit clinical manifestations except when exposed to a high number of pathogenic mycobacteria.10 In dogs that develop clinical signs, the most common primary sites of TB infection are the lungs and the pulmonary lymph nodes, being pneumonia the main clinical manifestation of M. tuberculosis.1,4,11 Nevertheless, occasional cases of canine mycobacterial infection involving primarily the digestive tract have been described worldwide, although some of them caused by non-tuberculous mycobacteria, especially M. avium.8,9 A generalized form of TB is considered rare in dogs infected with M. tuberculosis.12 These cases probably result from hematogenous dissemination of bacilli following erosion of the wall of a blood vessel by an expanding tubercle. In some instances, presumably following substantial release of bacilli into the blood, the presence of innumerable tiny white foci justifies the term “miliary tuberculosis.” Embolic lesions are most common in lung, and may involve lymph nodes, bone, liver, kidney, mammary gland, uterus, pleura, peritoneum, pericardium, and meninges. Lesions are rare in salivary gland, pancreas, spleen, brain, myocardium, or muscle.2,3 Unlike other species, such as bovine, TB in dogs often appears as granulation tissue in which macrophages are scattered at random and giant cells are rare. Small and generalized granulomas are uncommon, and are composed principally of epithelioid cells surrounded by narrow zones of fibrous tissue in which there are scattered small collections of lymphocytes and plasma cells. Thus, the lack of Langhans cells and demarcation of the granulomas is considered as an essential feature of TB in dogs. In addition, the necrosis is not a feature in these kind of granulomas but is often present in the centers of larger and more chronic ones.2 In dogs, intrabronchial dissemination within the lungs occurs quite rapidly and can lead to tuberculous bronchitis and bronchiolitis. Pleuritis or peritonitis often accompanies primary infections in the lungs or intestine, respectively, with diffuse or finely nodular pleural thickening by granulation tissue containing few macrophages and bacilli.2
Dogs can also be infected by non-tuberculous mycobacteria which, in contrast, are ubiquitous and potentially pathogenic. Within the nontuberculous mycobacteria group, the Mycobacterium avium complex (MAC) encompasses mycobacterial species considered to be the most likely causative agent of disseminated disease in humans and dogs. Zoonotic transmission of MAC is as likely as environmental acquisition.3 Dogs are relatively less susceptible to infection with MAC organisms than to MTC pathogens, and despite the ubiquitous and widespread nature of MAC organisms, infections in dogs are rare, owing to their innate resistance.6
Intradermal tuberculin testing and serological tests in dogs are considered inconsistent and unreliable. The pathomorphological and bacteriological analyses are still the most reliable tests and constitute mainly post-mortem diagnosis. Although PCR techniques are becoming very valuable, mycobacterial culture is considered the reference standard for TB diagnosis.1,3,11 The use of ZN staining in microscopic examination, cytology, and/or histology samples for the identification of acid-fast organisms is a valuable method for routine mycobacterial diagnosis. In this case, the presence of acid-fast bacteria in the granulomas observed was not homogeneous with ZN stain. Very few or absence of bacteria were observed in the most chronic granulomas in which areas of central necrosis are seen but higher amounts of them were seen in the more incipient granulomas, in which there are not areas of evident central necrosis. This fact can justify that bacteria were not observed in biopsies taken antemortem with Zn stain. These results demonstrate the possibility of obtaining false negative results using ZN and show the need for combining methods in the diagnosis of canine TB.11
Contributing Institution: Servei de Diagnostic de Patologia Veterinària, Facultat de Veterinària UAB, Bellaterra (Barcelona).
JPC Diagnosis: Kidney: Nephritis, granulomatous, multifocal to coalescing, marked.
JPC Comment: The hallmark of Mycobacterium tuberculosis (MTB) infection is the pulmonary granuloma; this case illustrates these classical caseous granulomas in an extrapulmonary location, and the contributor does an excellent job summarizing this disease in dogs.
A recent article by Pereira et al in the Journal of Comparative Pathology described the histologic appearance and distribution of MTB lesions in naturally infected fifteen nonhuman primates, and the authors demonstrated that the infection did not always manifest as pulmonary granuloma.12 In all nine infected Old World Monkeys (OWMs), granulomas were present in at least one organ, with six having typical pulmonary granulomas.12 These granulomas were either caseous (with a necrotic core), non-necrotizing (solidly-cellular), or suppurative.12
In New World Monkeys (NWMs), on the other hand, pulmonary granulomas were only observed in one of six infected animals.12 Four other infected NWMs had diffuse interstitial pneumonia with foamy macrophages expanding alveolar septae but without distinct granulomas or necrosis.12 This macrophage morphology may be due to accumulation of MTB lipids in vesicles, and foam cell formation may be induced by oxygenated forms of mycolic acid. Only one NWM, an Uta Hick’s bearded saki, had typical pulmonary granulomas.
In this case, conference participants discussed the variable appearance of the lesions in the kidney: some are mature granulomas, while others are poorly organized and likely reflect an earlier change. Conference participants also discussed the spread of these lesions: while arrival at the kidney was likely hematogenous given the multisystemic distribution, spread within the kidney may have occurred through blood vessels or within affected tubules. Ultimately, participants decided the route of spread could not be determined in this histologic section.
References
- Bonovska, M., Tzvetkov, Y., Najdenski, H., Bachvarova, Y. PCR for detection of Mycobacterium tuberculosis in experimentally infected dogs. J Vet Med B Infect Dis Vet Public Health. 2005; 52(4): 165-170.
- Caswell JL, Williams KJ. Respiratory System, In: Maxie MG, ed. Jubb Kennedy and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. St. Louis, MO: Elsevier Press. 2016: 547-551.
- De la Fuente C, Pumarola M, Ródenas S, Foradada L, Lloret A, Pérez De Val B, Anor S. Imaging diagnosis-magnetic resonance imaging findings of an intracranial epidural tuberculoma in a dog. Vet Radiol Ultrasound. 2012;53: 655-659.
- Engelmann N, Ondreka N, Michalik J, Neiger R. Intra-abdominal Mycobacterium tuberculosis infection in a dog. J Vet Intern Med. 2014; 28:934-8.
- Erwin PC, Bemis DA, Mawby DI, et al. Mycobacterium tuberculosis transmission from human to canine. Emerg Infect Dis. 2004; 10(12): 2258-2260.
- Etienne C, Granat F, Trumel C, et al. A mycobacterial coinfection in a dog suspected on blood smear. Vet Clin Pathol. 2013; 42:516-21.
- Greene CE, et al. Mycobacterial infections. In: Greene EC, Gunn-Moore AD, eds. Infectious Diseases in Dogs and Cats. Vol 1. 2nd Mexico: McGraw-Hill Interamericana. 2000:246-258.
- Greene CE, Prescott JF. Mycobacterial infections. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 3rd St. Louis, MO: Elsevier. 2012; 334-335.
- Horn B, Forshaw D, Cousins D, Irwin PJ. Disseminated Mycobacterium avium infection in a dog with chronic diarrhea. Aust Vet J. 2000; 78: 320-325.
- LoBue PA, Enarson DA, Thoen CO. Tuberculosis in humans and animals: an overview. Int J Tuberc Lung Dis. 2010; 14: 1075-1078.
- Martinho AP, Franco MM, Ribeiro MG, et al. Disseminated Mycobacterium tuberculosis infection in a dog. Am J Trop. Med Hyg. 2013; 88:596-600.
- Pereira AHB, Lopes CAA, Pissinatti TA, et al. Pulmonary Granuloma Is Not Always the Tuberculosis Hallmark: Pathology of Tuberculosis Stages in New World and Old World Monkeys Naturally Infected with the Mycobacterium tuberculosis J Comp Path. 2022; 199: 55-74.
- Ribeiro, M. G. et al. Pre−Multidrug-Resistant Mycobacterium tuberculosis Infection Causing Fatal Enteric Disease in a Dog from a Family with History of Human Tuberculosis. Emerg. Dis. 2016; 64: e4-e7.
- Snider, WR. Tuberculosis in canine and feline populations. Review of the literature. Am Rev Respir Dis. 1971: 104(6), 877-887.
- Szalus?-Jordanow, O., Augustynowicz-Kopec?, E., Czopowicz, M., et al. Intracardiac tuberculomas caused by Mycobacterium tuberculosis in a dog. BMC Veterinary Res. 2016; 12(1).
- Turinelli V, Ledieu D, Guilbaud L, et al. Mycobacterium tuberculosis infection in a dog from Africa. Vet Clin Path. 2004; 33:177-181.
- Une Y, Mori T. Tuberculosis as a zoonosis from a veterinary perspective. Comp Immunol Microbiol Infect Dis. 30, 415-425.