Signalment:
28-year-old, female, Nilgiri langur, (Trachypithecus johnii).An adult female
captive born Nilgiri langur (
Trachypithecus johnii) from a zoological
garden in Central Europe developed an edematous swelling of the left thigh,
which persisted for several months and was associated with periods of a
decreased general condition, depression, and anorexia. Sonographic examination
of the thorax and abdomen revealed cardiomegaly as well as poor demarcation and
cloudy appearance of the liver. The animal was finally euthanized due to a poor
general condition, anorexia, and therapeutic resistance.
Gross Description:
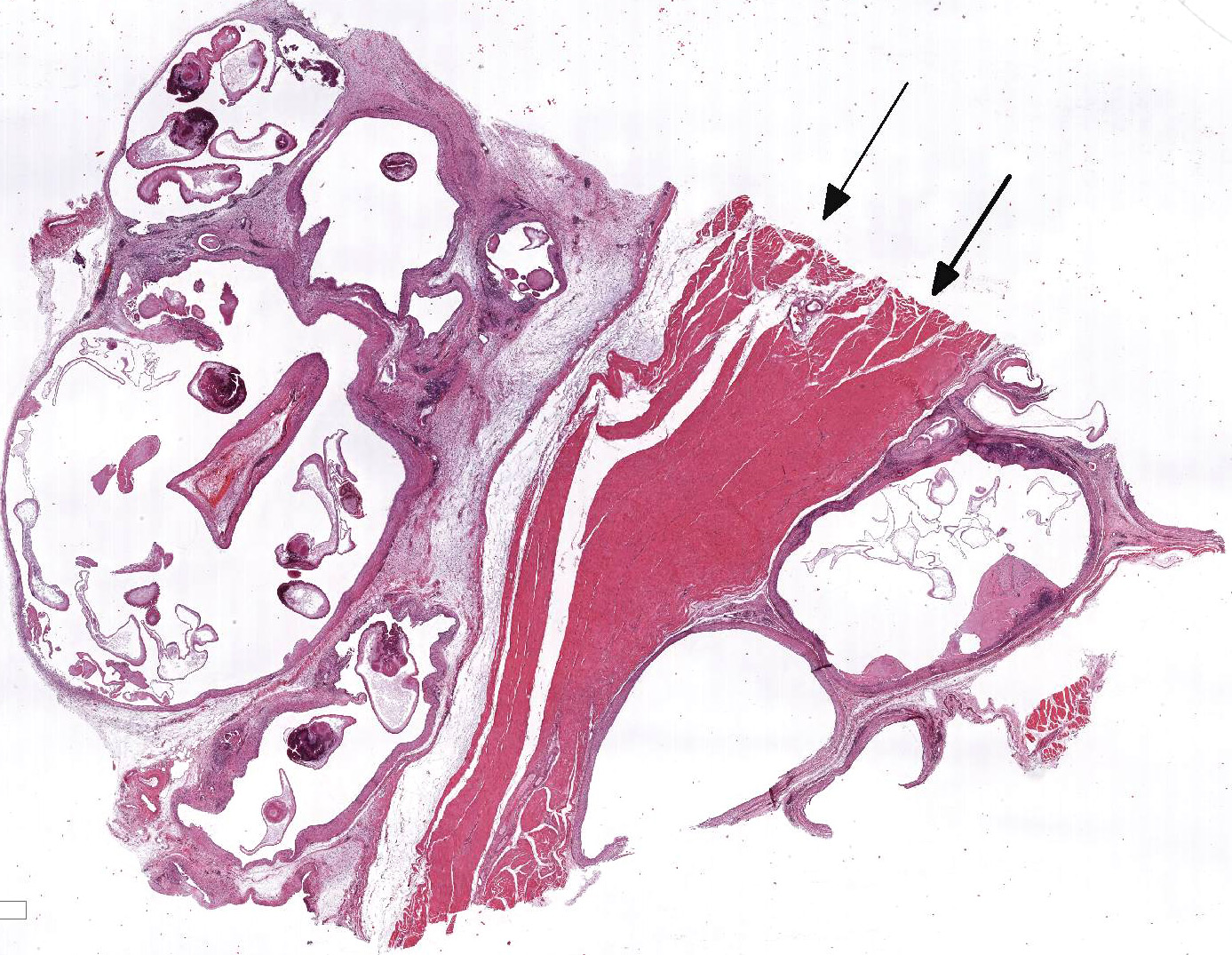
At necropsy, the
animal was cachectic. The skeletal muscle of the left thigh was severely
atrophic and replaced by fluctuant multilocular cysts containing numerous sand
grain sized whitish structures. The left caudal lung lobe revealed a focal
circumscribed area of atelectasis.
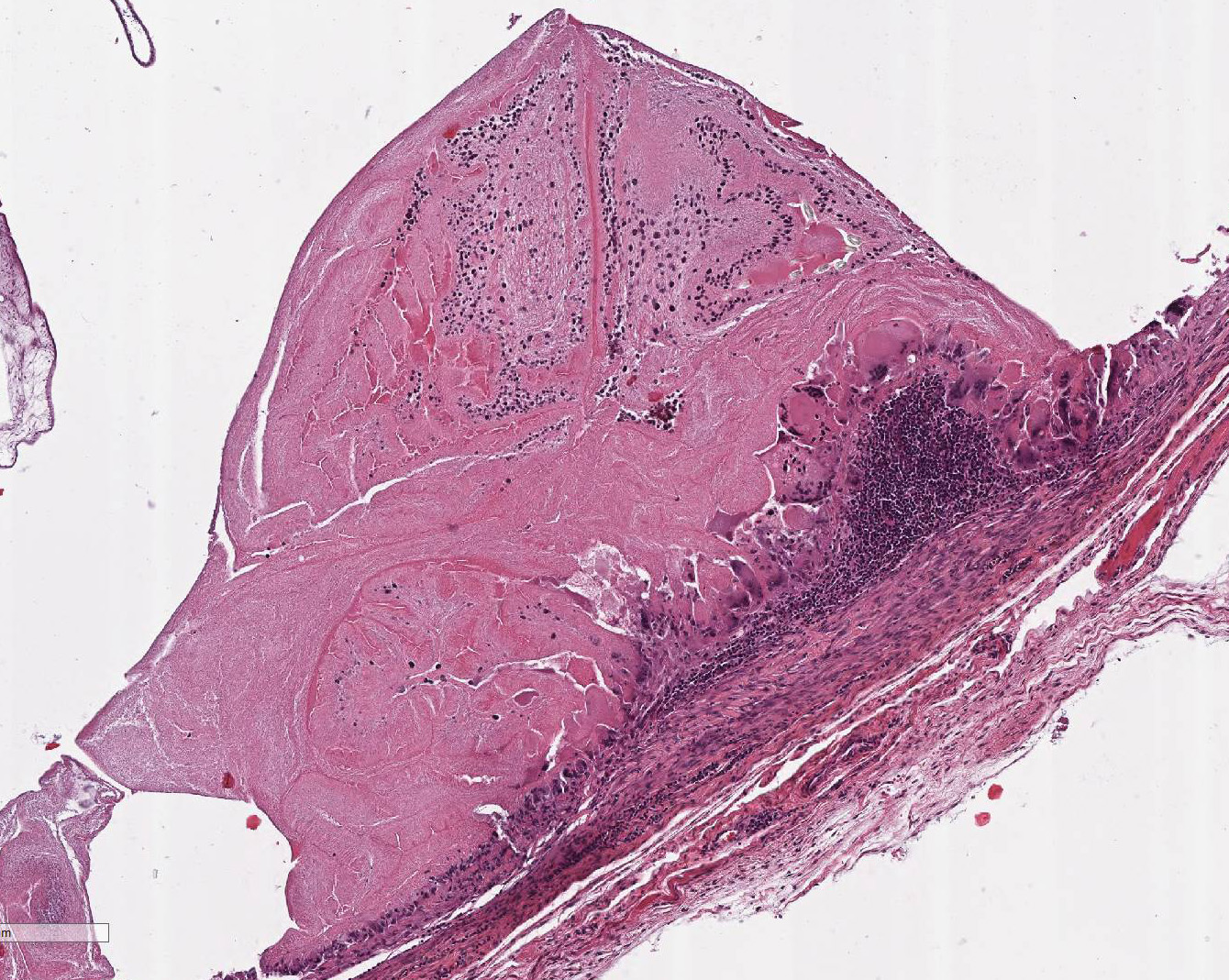
Histopathologic Description:
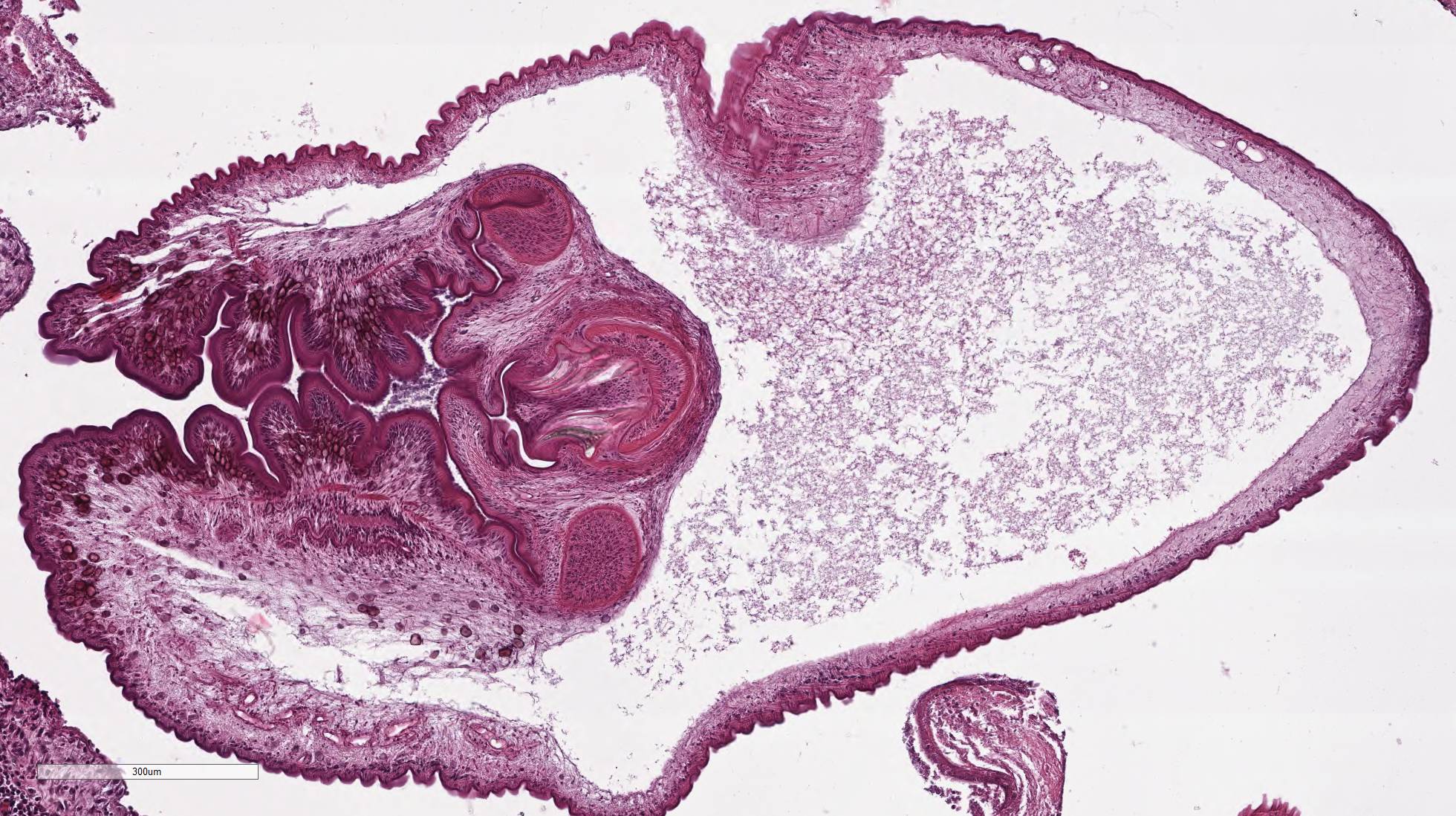
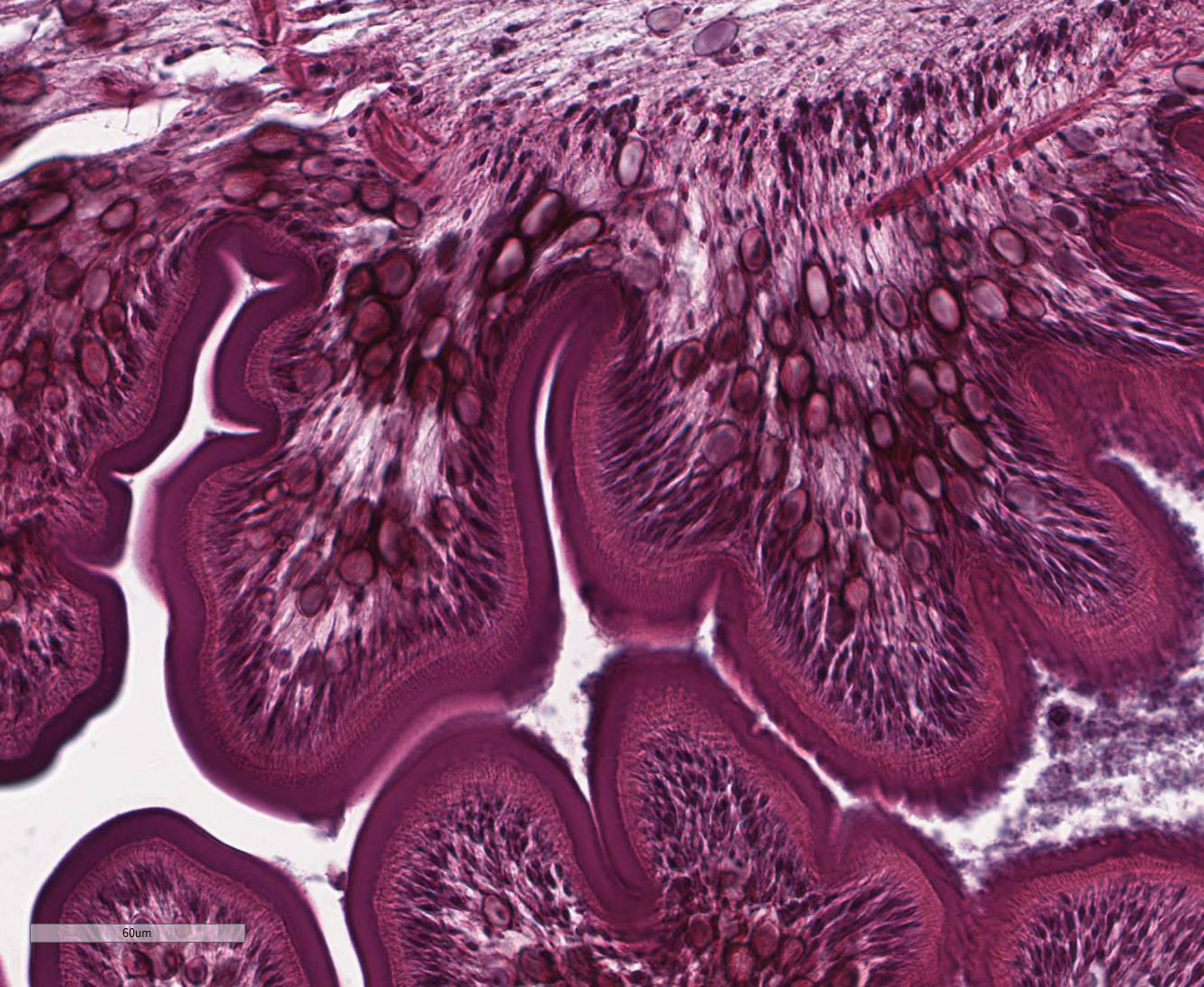
Within the skeletal muscle of the left
thigh are multifocal extensive areas of fibrous connective tissue bearing
multiple cystic structures with numerous larval cestodes (cysticerci). Cysts
are surrounded by thick fibrous capsules that are multifocally infiltrated by
plasma cells, lymphocytes, macrophages, and eosinophils. The inflammatory cells
extend into the adjacent fibrous granulation tissue between muscle fibers that
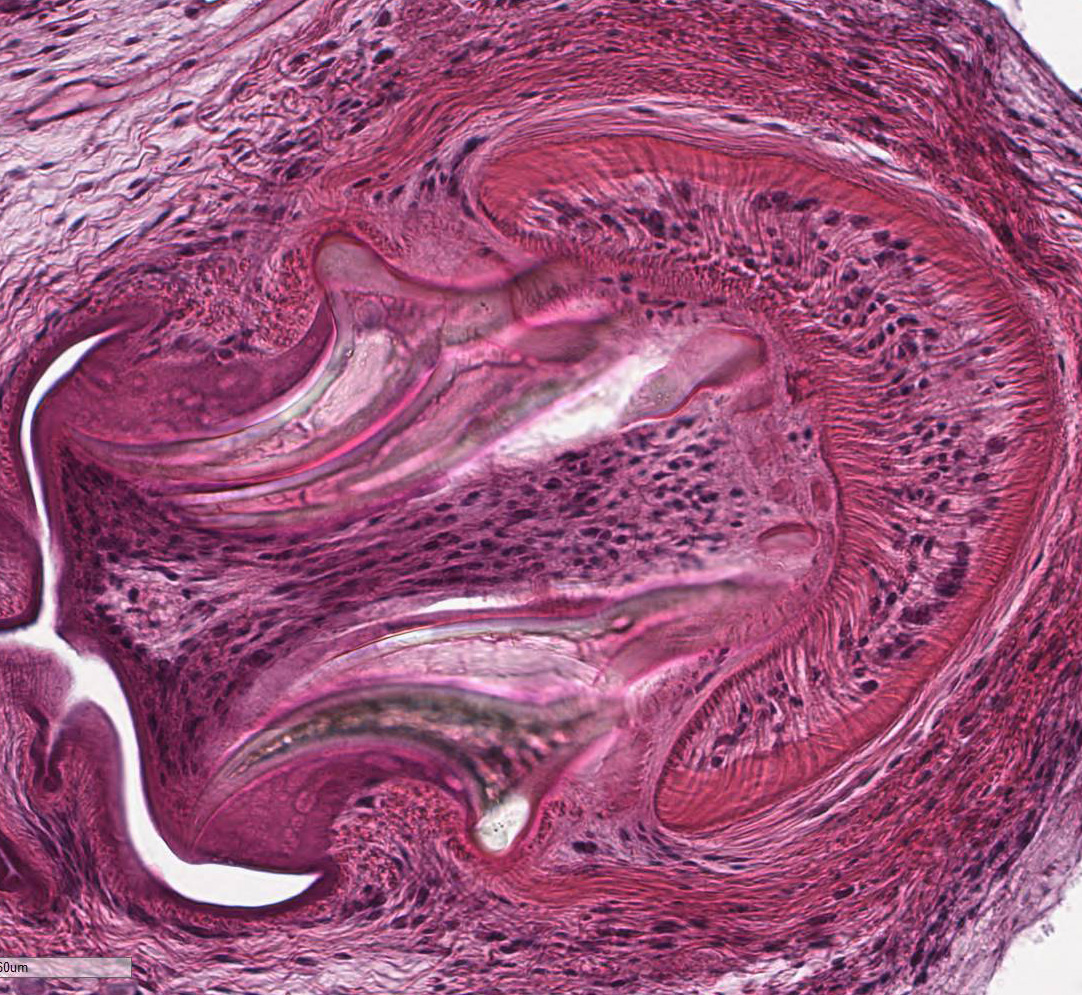
contains few multinucleated giant cells. Cysticerci are characterized by a 4 µm
thick, eosinophilic tegument, a fibrillar, eosinophilic parenchyma, numerous 5
µm diameter, basophilic, calcareous corpuscles, and an invaginated scolex with
muscular suckers and hooks.
Morphologic Diagnosis:
Skeletal muscle: Myositis, chronic, gran-ulomatous
and eosinophilic, multifocal, severe, with intralesional cysticerci, Nilgiri
langur (
Trachypithecus johnii), non-human primate.
Lab Results:
PCR
analysis of muscular metacestode tissue identified
Cysticercus longicollis,
the larval stage of
Taenia crassiceps,
as the etiologic agent.
Condition:
Cysticercus longicollis, langur
Contributor Comment:
Non-human
primates might act as aberrant hosts for a number of cestode species after oral
infection and larval development in extra-intestinal locations.
2 Taenia
crassiceps is a cestode parasite of the Northern hemisphere, whose life
cycle includes canids as definitive hosts, most commonly the red fox (
Vulpes
vulpes) in Europe and the Artic fox (
Alopex lagopus) as well as the
red fox in North America.
6 Natural infection by
T. crassiceps
has also been reported in wolves (
Canis lupus)
5 and coyotes (
Canis
latrans)
13 in North America as well as in wild cats (
Felis
silvestris)
12 and domestic dogs in Germany.
3 Several
rodent species and rabbits serve as intermediate hosts for the metacestode
larval stage of the parasite,
Cysticerus (
C.)
longicollis.
However, the common vole (
Microtus arvalis) is the predominant
intermediate host in Europe.
1 Sporadic cases of clinical
cysticercosis caused by
T. crassiceps have been reported in
humans and domestic animals such as dogs and cats, many of them in immuno-compromised
individuals.
8,9,16 Infections by
T. crassiceps may be
particularly serious due to their proliferative nature. In contrast to
cysticercosis associated with other
Taenia species,
T. crassiceps
is able to proliferate by exogenous and endogenous budding. Exogenous budding
may produce 1-6 daughter cysticerci at the abscolex pole of the maternal cyst.
Daughter cysticerci may bud off or remain attached by a stalk, form a scolex of
their own, and bud again. Endogenous budding occurs less commonly and is seen
in larger, older cysticerci. Such reproductive capability may result in
extensive infections, most frequently involving the subcutis and pleural and
peritoneal cavities. In humans, there are occasional reports about intraocular
mani-festations of cysticercosis.
4
In non-human
primates, there are documented cases of
T. crassiceps cysticercosis in a
black lemur (
Eulemur macaco macaco)
4 and in a ring-tailed
lemur (
Lemur catta)
10, both of them being prosimian species.
T.
crassiceps cysti-cercosis in an Old World monkey species like the Nilgiri
langur has not been reported before. Interestingly, in the langur, metacestode
tissue was not limited to the skeletal muscle, but could also be observed in
the left caudal lung lobe, reflecting the proliferative and invasive nature of
this parasite.
Other
Taenia
species causing cysticercosis in non-human primates include
Taenia solium,
Taenia crocutae,
Taenia hydatigena, and
Taenia martis.
2,14
However, infections with the larval cestodes mainly occur in Old World monkeys
and apes, while reports of taeniid cysticercosis in New World monkeys and
prosimians are sparse.
Studies
on the immune response elicited by
T. crassiceps and its antigens in
human and mice cells suggest a strong capacity of this parasite to induce a
chronic Th2-type response that is primarily characterized by high levels of Th2
cytokines, a low proliferative response in lymphocytic cells, an immature and
LPS-tolerogenic profile in dendritic cells, recruitment of myeloid-derived
suppressor cells, and by activated macrophages.
11
JPC Diagnosis:
Skeletal muscle: Cysticerci, multiple, with mild chronic granulo-matous
inflammation, Nilgiri langur (
Trachypithecus johnii).
Conference Comment:
The contributor provides a striking example of multiple
intramuscular cysticerci containing cross sections of taeniid metacestodes, the
larval form of cestode tapeworms. The class
Cestoda has two orders of
veterinary importance. The first is
Pseudophyllidea, comprised of
Diphyllobothrium
sp. and
Spirometra sp.
15 These parasites grow into extremely
large adults, up to 15 meters in humans, lack suckers, and require two
intermediate hosts, typically an aquatic copepod and fish. In contrast, the
order
Cyclophyllidae, which contains
Taeniidae,
Mesocestoididae,
Dipylidiidae,
Ano-plocephalidae, and
Hymenolepididae,
require only one intermediate host, usually a land mammal or arthropod.
15
Adult cestodes are normally present in the
intestine, hepatic ducts, and/or pancreas of the final definitive host while
the larval forms are present within the tissue or body cavities of intermediate
hosts. Adult cestodes are broken into segments, called proglottids, which
contain both female and male reproductive organs. Cyclophyllidae have four
anterior suckers present in both larval and adult cestodes and birefringent
armed hooks, depending on the species. The four anterior suckers may not all be
visible histologically due to varying planes of section.7,15
While adult tapeworms are usually of minor
significance in their carnivorous definitive hosts, the larval form can migrate
into various tissue and cause significant pathology in intermediate or
paratenic hosts. Conference participants discussed the four different forms of
larval cestodes in tissue section. These include cysticercus, present in this
case, strobilocercus, coenurus, and the hydatid cyst.7,15 The
cysticercus is thin walled, fluid filled, and contains a single larva with one
inverted scolex and four suckers. The strobilocercus is later in development
and contains an evaginated and elongated scolex and develops multiple segments,
similar to the adult cestode. Coenurus is similar to cysticercus but contains
more than one scolex, all of which can develop into an adult in the definitive
host. Hydatid cysts, typical of the genus Echinococcus sp., have a
bladder with large numbers of small protoscolicies grouped into clusters called brood capsules.7,15
The inflammatory response in this case is mild and composed
of a mixed population of histiocytes, multinucleated giant cell macrophages,
lymphocytes, and plasma cells with mild atrophy of the adjacent skeletal muscle
bundles. The glycoprotein-rich wall of the cysticerci provokes little to no
host reaction when intact; however, rupture of the cysticerci results in a
severe granulomatous inflammation, fibrosis, and mineralization.15
Additionally, Cysticercus longicollis, the larval form of Taenia
crassiceps present in this case, undergoes both endogenous and exogenous
budding of the cysticerci leading to severe and disseminated infection.4
References:
1. Bröjer
CM, Peregrine AS, Barker IK, et al. Cerebral cysticercosis in a woodchuck (Marmota
monax). J Wildl Dis. 2002;38:621-624.
2. Brunet
J, Pesson B, Chermette R, et al. First case of peritoneal cysticercosis
in a non-human primate host (Macaca tonkeana) due to Taenia martis.
Parasit Vectors. 2014;7:422.
3. Dyachenko
V, Pantchev N, Gawlowska S, et al. Echinococcus multilocularis infections
in domestic dogs and cats from Germany and other European countries. Vet Parasitol.
2008;157:244-253.
4. Dyer
NW, Greve JH. Demar M, et al. Severe Cysticercus longicollis
cysticercosis in a black lemur (Eulemur macaco macaco). J Vet
Diagn Invest. 1998;10:362-364.
5. Freeman
RS. Cestodes of wolves, coyotes and coyote-dog hybrids in Ontario. Can J
Zool. 1961;39:527-532.
6. Freeman
RS. Studies on the biology of Taenia crassiceps (Zeder, 1800) Rudolphi,
1810 (Cestoda). Can J Zool. 1962;40:969-990.
7. Gardiner CH and Poynton SL: Morphologic
characteristics of cestodes in tissue section. In: An atlas of metazoan parasites in
animal tissues: American Registry of Pathology, Washington, D.C. 1999,
50-55.
8. Hoberg
EP, Ebinger W, Render JA. Fatal cysticercosis by Taenia crassiceps (Cyclophyllidea:
Taeniidae) in a presumed immunocompromised canine host. J Parasitol.
1999;85:1174-1178.
9. Lescano
AG, Zunt J. Other cestodes: sparganosis, coenurosis and Taenia crassiceps cysticercosis.
Handb Clin Neurol. 2013;114:335-345.
10. Luzón
M, de la Fuente-López C, Martínez-Nevado E, et al. Taenia
crassiceps cysticercosis in a ring-tailed lemur (Lemur catta). Zoo Wildl Med.
2010;41:327-330.
11. Peón
AN, Espinoza-Jiménez A, Terrazas LI. Immunoregulation by Taenia crassiceps
and its antigens. Biomed Res Internat. 2013. doi: 10.1155/2013/498583.
12. Schuster
R, Heidecke D, Schierhorn K. Contribution to the parasite fauna of local hosts.
On the endoparasitic fauna of Felis silvestris. Appl. Parasitol.
1993;34:113-120.
13. Seesee
FM, Sterner MC, Worley DE. Helminths of the coyote (Canis latrans Say)
in Montana. J Wildl Dis. 1983;19:54-55.
14. Strait
K, Else JG, Eberhard ML. Parasitic diseases of nonhuman primates. In: Abee CR,
Mansfield K, Tardif S, Morris T, eds. 2nd ed. Nonhuman
primates in biomedical research: diseases. San Diego,
USA: Academic Press; 2012:197-298.
15. Uzal
FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG ed.
In: Jubb Kennedy and Palmer's Pathology of Domestic Animals. Vol
2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:221-225.
16. Wüschmann
A, Garlie V, Averbeck G, et al. Cerebral cysticercosis by Taenia crassiceps
in a domestic cat. J Vet Diagn Invest. 2003;15:484-488.