CASE 2: M17-18662 (4118631-00)
Signalment: 12-14 weeks old, unknown gender and breed, pig (Sus scrofa domesticus)
History:
This piggery had 33 breeding sows, several boars, including three introductions within the last 12 months, and home-bred piglets, weaners and growers. Under temporary new management approximately 50 weaners had died in the past 6 months, following intermittent bouts of inappetence, diarrhoea, lethargy and wasting. Sows were up to date with vaccinations for erysipelas, leptospirosis and parvovirus, and all animals were being fed age-appropriate commercial feed. The property had issues with rodent control and flooding in the weaner shed. Samples were submitted from weaners, including one dead (pig 1) and two euthanized (pigs 2 & 3), that had diarrhoeic faeces containing undigested feed, and were weak and wasted.
Gross Pathology:
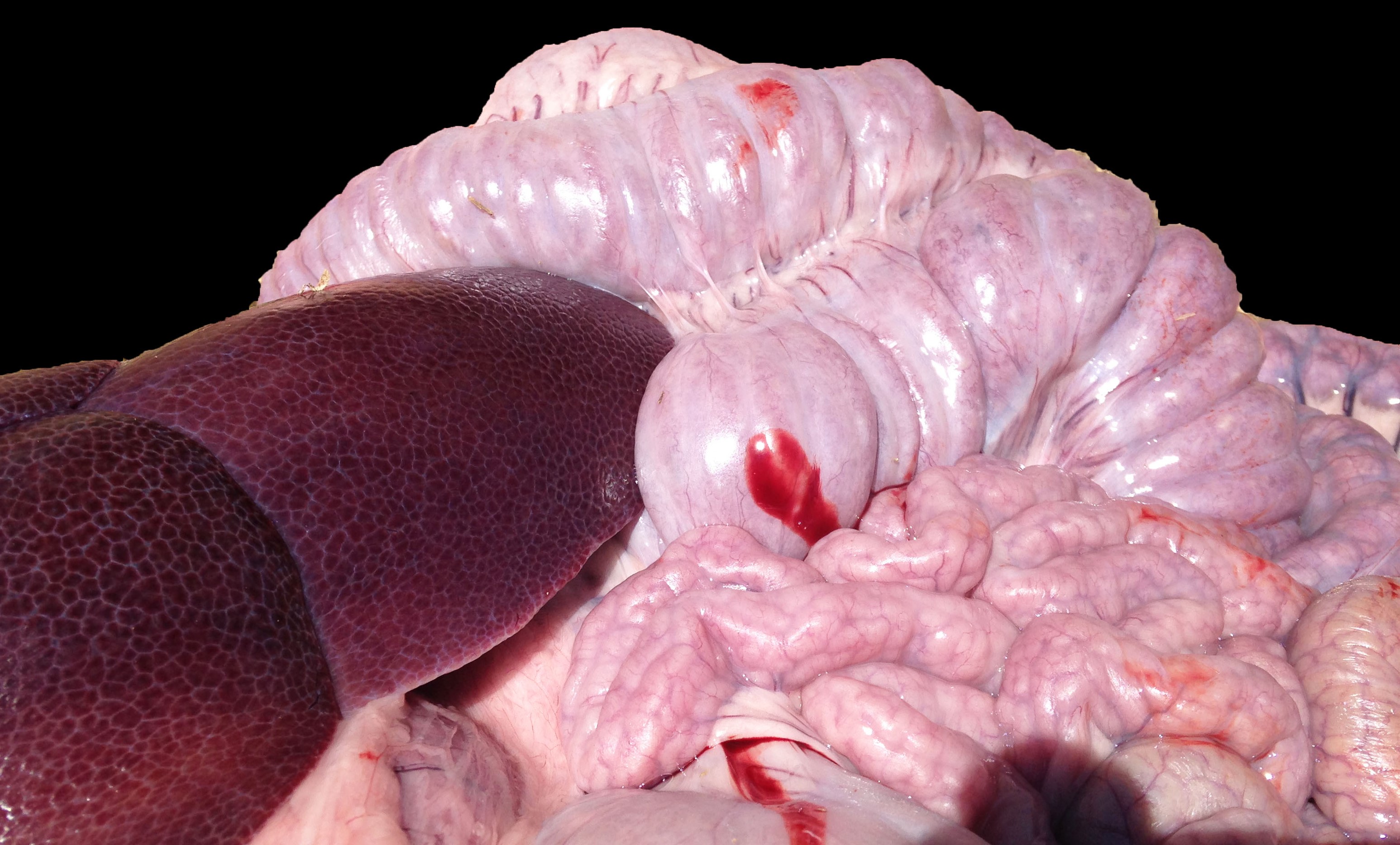
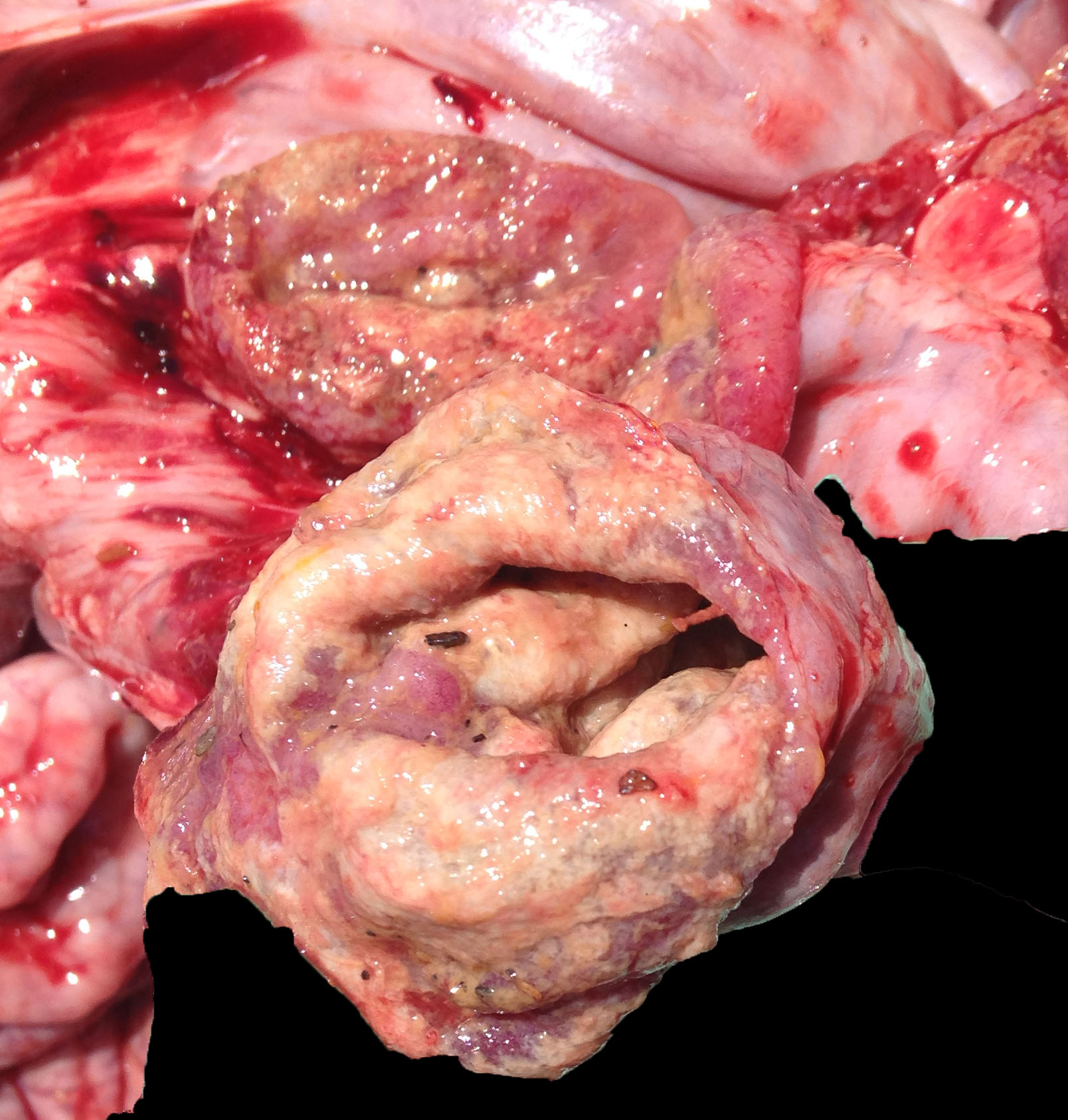
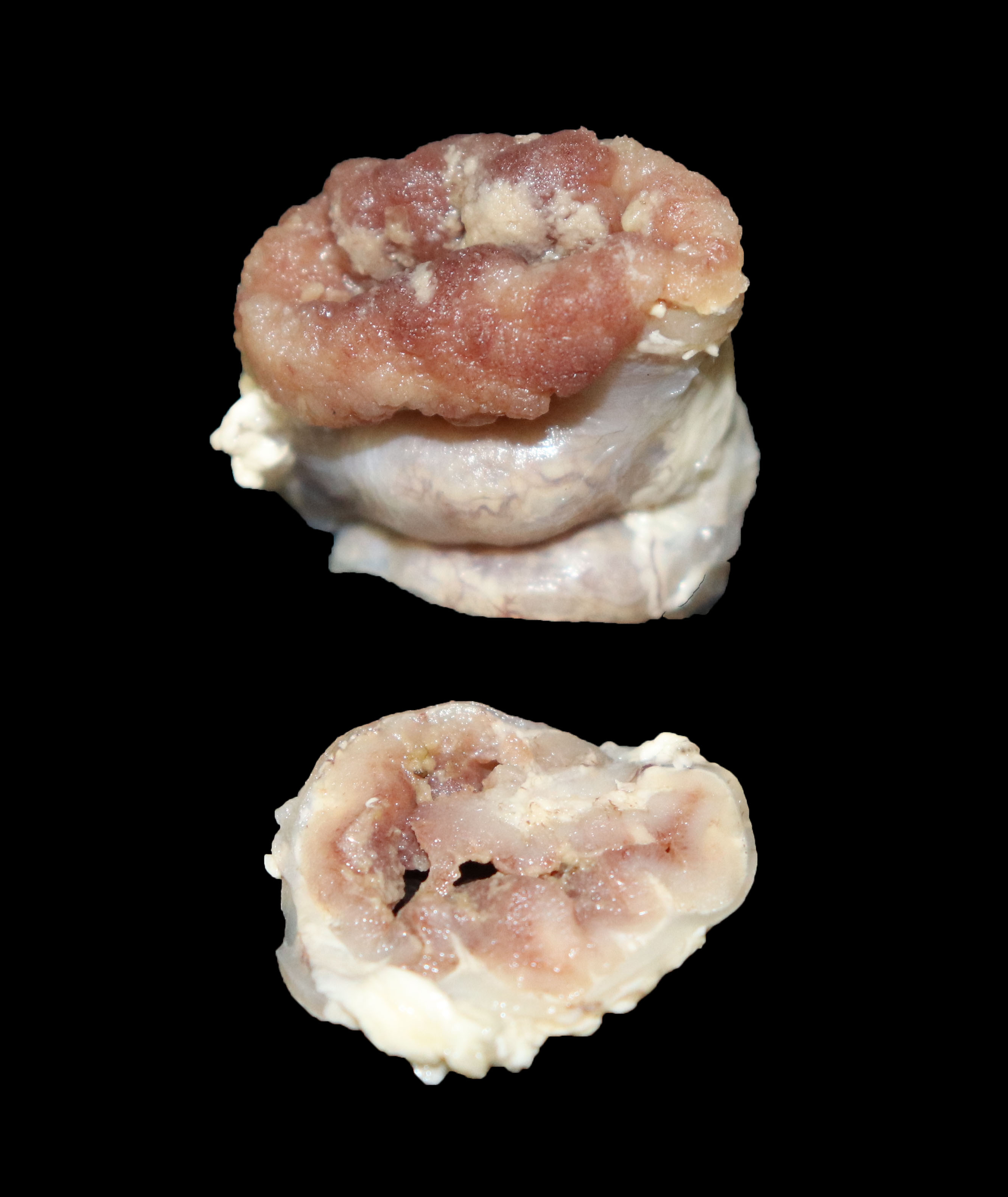
The small and large intestines in all three weaners were grossly thickened and were lined by diphtheritic membranes in pig 3.
Laboratory results:
PCR of faeces (pigs 1 and 2) was positive for Lawsonia intracellularis (LI) and Brachyspira pilosicoli. Samples submitted between pigs 1 and 2 were selective salmonella culture was negative, PCR was negative for Brachyspira hyodysenteriae, porcine delta coronavirus (PDCoV), porcine epidemic diarrhoea virus (PEDV), transmissible gastroenteritis virus (TGE), porcine respiratory coronaviruses, bovine viral diarrhoea virus (BVDV), classical swine fever (CSF) and African swine fever (ASF), and general mammalian virus isolation did not show any cytopathic effect. Blood was porcine circovirus 2 (PCV2) rt-PCR low positive at a level that was not consistent with porcine circovirus associated disease (PCVAD).
Microscopic description:
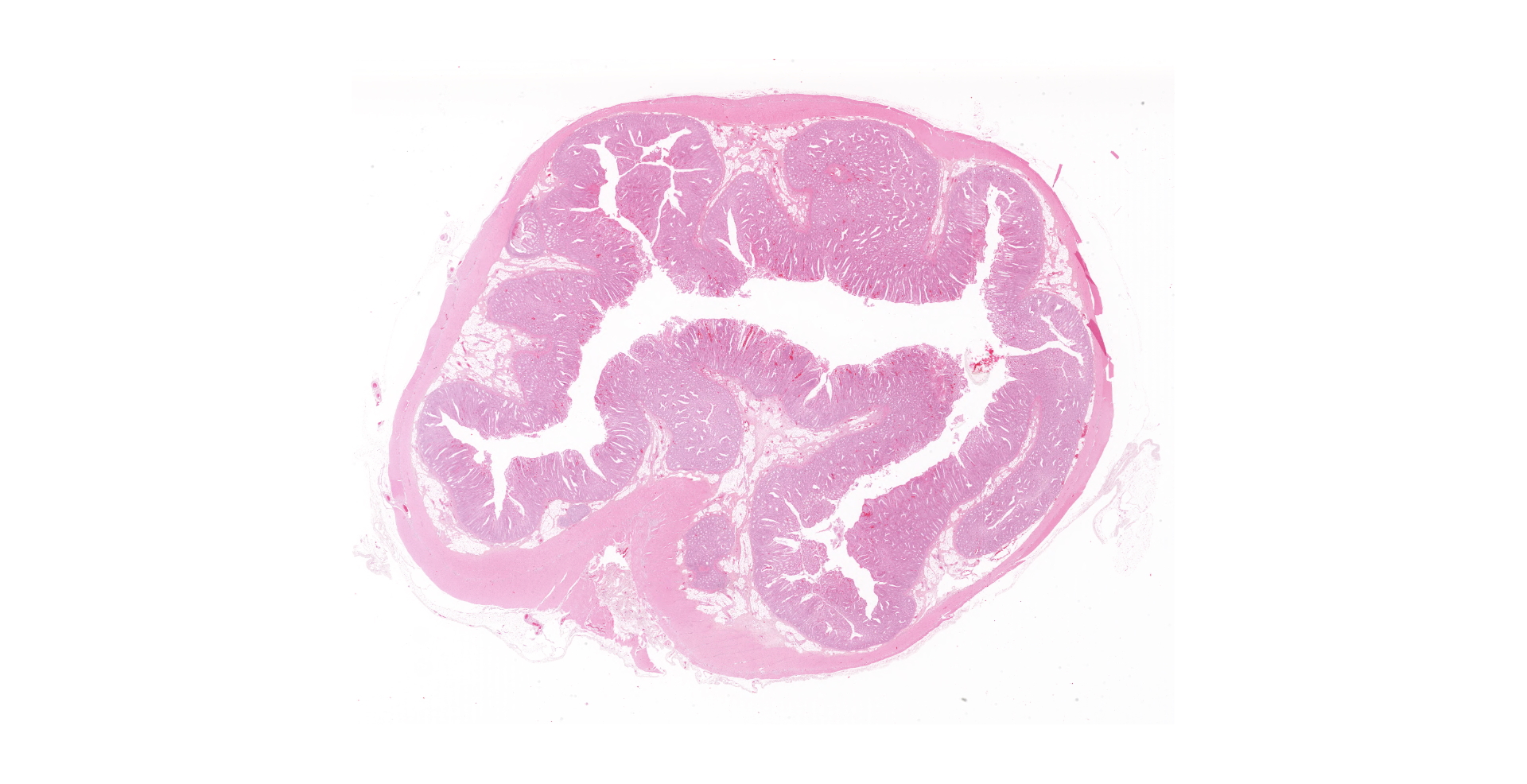
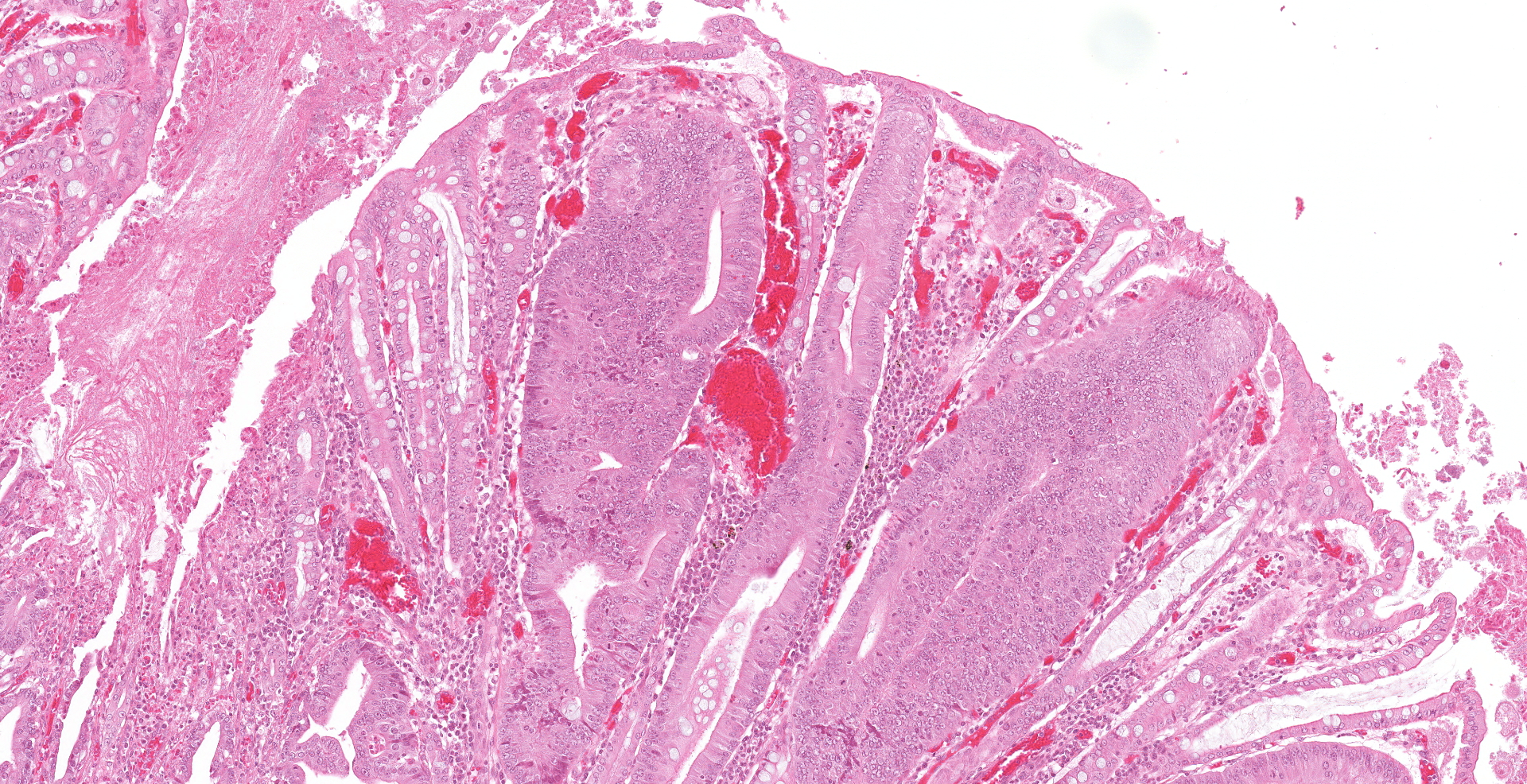
One unlabelled short segment of intestine was examined (pig 3). Differentiation between distal ileum, caecum and colon is difficult due to extensive pathology present.
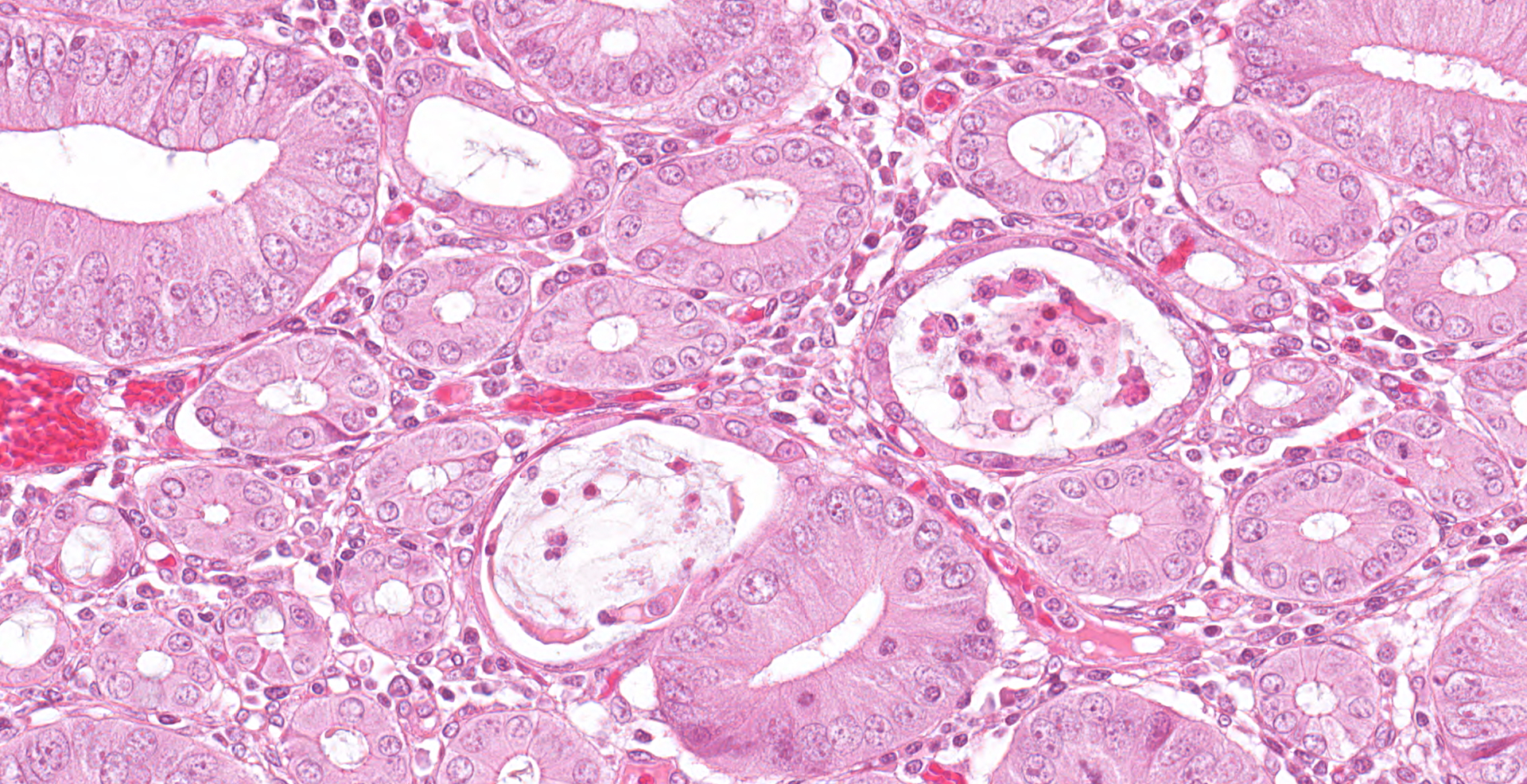
The mucosa is diffusely markedly thickened by pseudo-stratified, crowded and mitotically active crypt epithelium, with plump, vesiculated nuclei. Multifocally, villous tips are moderately to severely blunted and fused, or replaced by thick bands of cellular and karyorrhectic debris (necrosis), admixed with granular, lightly basophilic material (bacterial colonies), moderate infiltrations of lymphocytes, plasma cells, neutrophils and fewer eosinophils, and moderate amounts of hypereosinophilic, fibrillar material (fibrin). The mucosa is concurrently expanded by clear space (edema) and mild lymphoplasmacytic proprial infiltrates. Crypt lumens are often expanded by mucinous material and cellular debris (cryptitis). Foci of adenomatous epithelium sometimes herniate into depleted germinal centres of gut associated lymphoid follicles (Peyer's patches). Variably between sections there are numerous intraluminal, or occasionally proprially invasive, large (30-54 x 36-76 um), rounded, ciliated protozoa, with a paracentral macronucleus (consistent with Balantidium coli trophozoites).
Histochemical stains: Warthin-Starry Silver stain reveals large numbers of short, comma-shaped, argyrophilic bacteria, clustered apically within enterocytes, free within crypt lumens and occasionally within B. coli trophozoites (consistent with L. intracellularis).
Immunohistochemistry (IHC): L. intracellularis: There was strong intracellular immunoreactivity at the apical margin of epithelial cells, and to a lesser extent within small numbers of macrophages in the lamina propria. B. pilosicoli and PCV2: There was no significant immunoreactivity within examined sections.
Contributor's morphologic diagnosis:
Intestine: enteritis, proliferative and fibrinonecrotizing, diffuse, severe, chronic, with lymphoid depletion and crypt herniation, cryptitis, intraepithelial, argyrophilic bacteria, aetiology consistent with L. intracellularis, and intralesional ciliated protozoal organisms, pig (Sus scrofa domesticus)
Contributor comments: Investigating the pri-mary cause of diarrhoea and wasting in growing pigs is complicated by mixed infections, as in this case, where histopathology was used to diagnose porcine proliferative enteropathy (PPE), caused by LI, with background B. pilosicoli and PCV-2 infections.
LI is an obligate intracellular, microaerophilic, non-flagellated, non-spore forming, gram-negative, curved or S-shaped rod bacterium.10,20-22 LI can infect a range of species including swine and foals, and less commonly donkeys, deer, sheep, rodents (e.g. guinea pigs, hamsters, mice, rats), rabbits, foxes, dogs, ferrets, emus, ostriches and non-human primates.21,22
The two main clinical manifestations of LI infection in pigs are porcine intestinal adenomatosis (PIA) and porcine haemorrhagic enteropathy (PHE). PIA is most common in post-weaning animals aged 2-5 months, and can progress to necrotic enteritis (NE), usually involving superimposed secondary bacterial infections, and then regional ileitis (RI) if the animal recovers from NE.6,10,20-22 PHE is acute and most common in finishing pigs, young gilts and boars, typically 4-12 months of age, especially when naïve animals are introduced to a site of endemic LI infection.6,22 Clinical signs of PIA vary from subclinical infection, to reduced growth rate with capricious appetite, to persistent diarrhoea, wasting and mortality,18,20,21 with mortality rates usually being low and most often a consequence of secondary bacterial infections.6 Main differential diagnoses include brachyspiral colitis, salmonellosis, colibacillosis, Clostridium perfringens type C, yersiniosis, coronaviruses and porcine circovirus-associated disease (PCVAD).9,17,19,22 PHE is associated with acute to subacute intestinal haemorrhage, which may manifest as melena or haematochezia, weakness and pallor, or rapid death associated with exsanguination, with mortality rates reaching up to 50%.6,20-22 The main differential diagnoses include swine dysentery (B. hyodysenteriae, B. hampsonii, and B. suanatina), salmonellosis, gastric ulcers and haemorrhagic bowel syndrome, which is often attributed to intestinal volvulus.22
Macroscopic lesions in PPE are most common in the terminal ileum and also occur in the cecum and colon.20,21 Rarely infection may be restricted to the large intestine.8 Gross lesions consist of ridges or plaque-like thickened areas that project above the normal mucosa and on the serosal surface the hyperplastic mucosa and edematous submucosa forms a reticular or cerebriform pattern of projections, which is virtually pathognomonic.20-22 In NE there is more extensive mucosal necrosis, often with diphtheritic membranes and luminal fibrin casts.9,20-22 PHE is characterised by overt or discrete areas of haemorrhage and ulceration, most common in the ileum.20,21
Following oral inoculation LI remains viable during passage through the stomach by utilizing systems that maintain pH homeostasis,22 and within 12 hours can be demonstrated in mature villous tip enterocytes.3,22 Within 5 days post inoculation the crypt epithelium becomes infested.3,22 The reasons for its bias for the ileum are unknown, but it may relate to specific receptors, a favourable physiological environment, or mechanical reasons such as gut transit time.22 Virulence factors associated with adhesion and entry have not been fully characterised, however the process is most likely dependent on host cell activity,22 and probably involves a type 3 secretion system (T3SS), the genes for which are present in porcine LI isolates.22
There have been several proposed mechanisms of LI intracellular survival, including a Salmonella pathogenicity island 2 (SPI2)-related operon which may enable escape from endosome into cytosol, a sophisticated oxidative protection mechanism, and an ATP/ADP translocase used to exchange bacterial ADP for host cell ATP, thus exploiting the hosts energy pools.15,22 Following vacuolar escape LI replicates in apical cytoplasm by binary fission, a process thought only to occur in actively dividing cells.22 LI induces enterocyte proliferation,22 although the exact host cell upstream cellular pathways leading to PE are unknown,1,5 proposed mechanisms include the production of a cyclomodulin,22 and simultaneous induction of Notch-1 signalling and attenuation of beta-catenin/Wnt pathways.5 The latter may also be responsible for inhibiting the maturation of goblet cells,5 which have also been shown to express lower levels of mucin 'MUC2' with LI infection, possibly altering the mucus barrier and helping to facilitate cellular invasion.1 Eventually LI extrudes out of host cells or is released following apoptosis, then either infecting new enterocytes via the gut lumen, proprial ECM or possibly macrophages, or being shed in feces.3,6,8,19,20,22
The diarrhea observed in PPE is most likely due to loss of functional mucosal surface area, whereas wasting most likely relates to protein-losing enteropathy.14,21,22 As the mucosal thickening in PIA becomes more extensive there is coagulative necrosis of the mucosa, allowing for colonization by pathogenic anaerobic large bowel flora and the formation of diphtheritic membranes and fibrin casts.21 It is unclear if development of PHE relates to the host, infectious dose, virulence of the infecting strain and/or other coinfections.6 Experimental infection of gnotobiotic pigs has shown that PPE will not occur without undefined interactions with other gut bacteria, such as pathogenic E. coli and Salmonella sp.21,22
As in this case, multiple potential pathogens may occur in the same animals, and the relationships between PPE and other diseases are often complex, with LI causing alterations in the composition of the gut microbiome, and either directly or indirectly increasing the likelihood of coinfection with some pathogens such as Salmonella enterica, and decreasing the likelihood of others, such as PCV2.6,12,17 Although B. pilosicoli was identified by PCR in this case, clinical signs and gross changes were not suggestive of porcine intestinal spirochetosis, which typically presents as a watery to mucoid diarrhea associated with typhlocolitis.13,21 Histologic changes in this case were consist with LI, and not with B. pilosicoli which causes goblet cell hyperplasia, with organisms often forming a 'false brush border' of palisading upright bacteria perpendicular to enterocytes, or being observed within colonic crypts and goblet cells.21 A flagellated protozoan, consistent with Balantidium coli, was also observed in this case. B. coli is a facultative pathogen of the intestinal lumen, usually associated with subclinical infection.16 Following injury to the intestinal wall trophozoites may penetrate into the mucosa, usually in the cecum and colon, causing diarrhea, hematochezia and tenesmus.16,21 In one study LI was identified in small numbers inside B. coli trophozoites in PPE cases.7
PIA does not usually feature a prominent inflammatory response, which may in part be due to LI downregulating CD2 expression, thereby minimising T cell activation.21,22 In more chronically affected pigs, or in cases that progress to NE, more significant proprial mononuclear infiltrates may be observed.22 Proprial histiocytosis, as well as depletion and necrosis of Peyer's patches and giant cell formation, may be observed both in PPE and PCV2-associated enteritis.9 However the latter also differs from PPE histologically in many respects, including histiocytosis in lymphoid tissue, cytoplasmic inclusion bodies, and PCV2 IHC positive histiocytes in the lamina propria, submucosa, gut associated lymphoid tissue and within villous and crypt enterocytes.9
The gold standard for diagnosis of PPE is IHC using LI specific antibodies.4,22 Other methods of characterizing spirochetal organisms on histologic section include in situ hybridization and electron microscopy.6,20 Cultivation of LI is not routinely performed, and requires live tissues cultures.20,21 Feces or tissue samples can be used for PCR and fecal smears can also be tested using immunoperoxidase staining.4
Contributing Institution:
State Veterinary Diagnostic Laboratory (SVDL)
Elizabeth Macarthur Agricultural Institute
New South Wales
Australia
JPC diagnosis:
1. Cecum: Typhlitis, proliferative, diffuse, severe, with multifocal ulceration, crypt herniation, and crypt abscessation, unknown breed, porcine.
2. Cecum, mucosa: Ciliates, few, etiology consistent with Balantidium coli.
JPC comment:
The contributor provides an excellent narrative for this disease and covers the topic comprehensively. This discussion was about manifestations of L. intracellularis in pigs, but it can affect other species as well.
L. intracellularis is the causative agent of equine proliferative enteropathy (EPE) of weanling foals in Scandinavia, which results in thickening of the small intestinal wall, and the ileum in particular. Clinical signs were similar to porcine patients, and included lethargy, anorexia, pyrexia, peripheral edema, colic, and diarrhea, with associated clinicopathologic changes of hypoalbuminemia and leukocytosis. This may represent a situation unique to Denmark due to the proximity of the discussed horses to high density pig farming operations, and no specific strain analysis has been performed to date.2
While exact pathogenesis has been elusive, the inflammatory response of infected cells has recently been characterized. By comparing control and infected animals, differentially expressed genes were isolated, and correlated well with the severity of lesions observed. Some important upregulated genes are XDH (encodes xanthine dehydrogenase), MMP-7 (encodes matrix metalloproteinase-7), TGM2 (encodes transglutaminase-2), and OSM (encodes oncostatin M). Xanthine dehydrogenase is an important regulator of inflammatory pathways, and ultimately results in the production of reactive oxygen and reactive nitrogen species. MMP-7 is important for activating a-defensin antimicrobial peptides and is often found to be overexpressed in colorectal cancers, causing an induced cellular proliferation. Oncostatin M is a cytokine associated with diseases with chronic inflammation, and transglutaminase-2 is also linked to cellular proliferation. The changes induced by L. intracellularis have similarities with some cancers, but once the infection is resolved, there is resolution and not neoplastic growth.11
References:
1. Bengtsson RJ, MacIntyre N, Guthrie J, Wilson AD, Finlayson H, Matika O, et al.: Lawsonia intracellularis infection of intestinal crypt cells is associated with specific depletion of secreted MUC2 in goblet cells. Veterinary Immunology and Immunopathology 2015:168(1-2):61-67.
2. Bohlin AM, Olsen SN, Laursen SH, Ohman A, van Galen G. Lawsonia intracellularis associated equine proliferative enteropathy in Danish weanling foals. Acta Veterinaria Scandinavica. 2019;61(12).
3. Boutrup TS, Boesen HT, Boye M, Agerholm JS, Jensen TK: Early Pathogenesis in Porcine Proliferative Enteropathy caused by Lawsonia intracellularis. Journal of Comparative Pathology 2010:143(2-3):101-109.
4. Guedes RMC, Gebhart CJ, Winkelman NL, Mackie-Nuss RAC, Marsteller TA, Deen J: Comparison of different methods for diagnosis of porcine proliferative enteropathy. Canadian Journal of Veterinary Research-Revue Canadienne De Recherche Veterinaire 2002:66(2):99-107.
5. Huan YW, Bengtsson RJ, MacIntyre N, Guthrie J, Finlayson H, Smith SH, et al.: Lawsonia intracellularis exploits beta-catenin/Wnt and Notch signalling pathways during infection of intestinal crypt to alter cell homeostasis and promote cell proliferation. Plos One 2017:12(3):21.
6. Jacobson M, Fellstrom C, Jensen-Waern M: Porcine proliferative enteropathy: An important disease with questions remaining to be solved. Veterinary Journal 2010:184(3):264-268.
7. Jensen TK, Boesen HT, Vigre H, Boye M: Detection of Lawsonia intracellularis in Formalin-fixed Porcine Intestinal Tissue Samples: Comparison of Immunofluorescence and In-situ Hybridization, and Evaluation of the Effects of Controlled Autolysis. Journal of Comparative Pathology 2010:142(1):1-8.
8. Jensen TK, Christensen BB, Boye M: Lawsonia intracellularis infection in the large intestines of pigs. Apmis 2006:114(4):255-264.
9. Jensen TK, Vigre H, Svensmark B, Bille-Hansen V: Distinction between porcine circovirus type 2 enteritis and porcine proliferative enteropathy caused by Lawsonia intracellularis. Journal of Comparative Pathology 2006:135(4):176-182.
10. Kim J, Won G, Park S, Lee JH: Identification of Lawsonia intracellularis putative hemolysin protein A and characterization of its immunoreactivity. Veterinary Microbiology 2017:205:57-61.
11. Leite FL, Abrahante JE, Vasquez E, et al. A cell proliferation and inflammatory signature is induced by Lawsonia intracellularis infection in swine. mBio. 2019. 10:e01605-18.
12. Leite FLL, Singer RS, Ward T, Gebhart CJ, Isaacson RE: Vaccination Against Lawsonia intracellularis Decreases Shedding of Salmonella enterica serovar Typhimurium in Co-Infected Pigs and Alters the Gut Microbiome. Scientific Reports 2018:8:10.
13. Reiner G, Winkelmann M, Willems H: Prevalence of Lawsonia intracellularis, Brachyspira hyodysenteriae, and Brachyspira pilosicoli infection in hunted wild boars (Sus scrofa) in Germany. European Journal of Wildlife Research 2011:57(3):443-448.
14. Roerink F, Morgan CL, Knetter SM, Passat MH, Archibald AL, Ait-Ali T, et al.: A novel inactivated vaccine against Lawsonia intracellularis induces rapid induction of humoral immunity, reduction of bacterial shedding and provides robust gut barrier function. Vaccine 2018:36(11):1500-1508.
15. Schmitz-Esser S, Haferkamp I, Knab S, Penz T, Ast M, Kohl C, et al.: Lawsonia intracellularis contains a gene encoding a functional rickettsia-like ATP/ADP translocase for host exploitation. Journal of Bacteriology 2008:190(17):5746-5752.
16. Schubnell F, von Ah S, Graage R, Sydler T, Sidler X, Hadorn D, et al.: Occurrence, clinical involvement and zoonotic potential of endoparasites infecting Swiss pigs. Parasitology International 2016:65(6):618-624.
17. Stahl M, Kokotovic B, Hjulsager CK, Breum SO, Angen O: The use of quantitative PCR for identification and quantification of Brachyspira pilosicoli, Lawsonia intracellularis and Escherichia coli fimbrial types F4 and F18 in pig feces. Veterinary Microbiology 2011:151(3-4):307-314.
18. Stege H, Jensen TK, Moller K, Vestergaard K, Baekbo P, Jorsal SE: Infection dynamics of Lawsonia intracellularis in pig herds. Veterinary Microbiology 2004:104(3-4):197-206.
19. Szczotka A, Stadejek T, Zmudzki J, Nowak A, Osinski Z, Pejsak Z: Immunohistochemical detection of Lawsonia intracellularis in tissue sections from pigs. Polish Journal of Veterinary Sciences 2011:14(4):531-538.
20. Taylor D: Pig Diseases. Lennoxtown, Glasgow: Dr. D. J. Taylor, 2013.
21. Uzal FA, Plattner BL, Hostetter JM: Alimentary System. St Louis, Missouri: Elsevier, 2016.
22. Vannucci FA, Gebhart CJ: Recent Advances in Understanding the Pathogenesis of Lawsonia intracellularis Infections. Veterinary Pathology 2014:51(2):465-477.